Our Aim
In multiple sclerosis immune cells infiltrate brain and spinal cord where they damage neurons and glial cells. This structural damage to the nervous system is responsible for the irreversible functional deficits that patients acquire over time. In our work we try to understand how immune cells damage the nervous, how reciprocal interactions between immune and nervous system control the formation and resolution of such inflammtory reactions and how we can best leverage this knowledge to design therapeutic approaches that prevent or limit tissue damage in multiple sclerosis.
Our Approach
We develop in vivo imaging techniques to visualize in real time the cellular and molecular interactions that underlie tissue damage in multiple sclerosis models, employ CRISPR-based gene editing to resolve the signaling pathways that govern this interactions and use genetic and pharmacological manipulations to evaluate new therapeutic strategies that foster tissue protection and repair.
Our Projects
The following aspects are of particular interest to our lab:
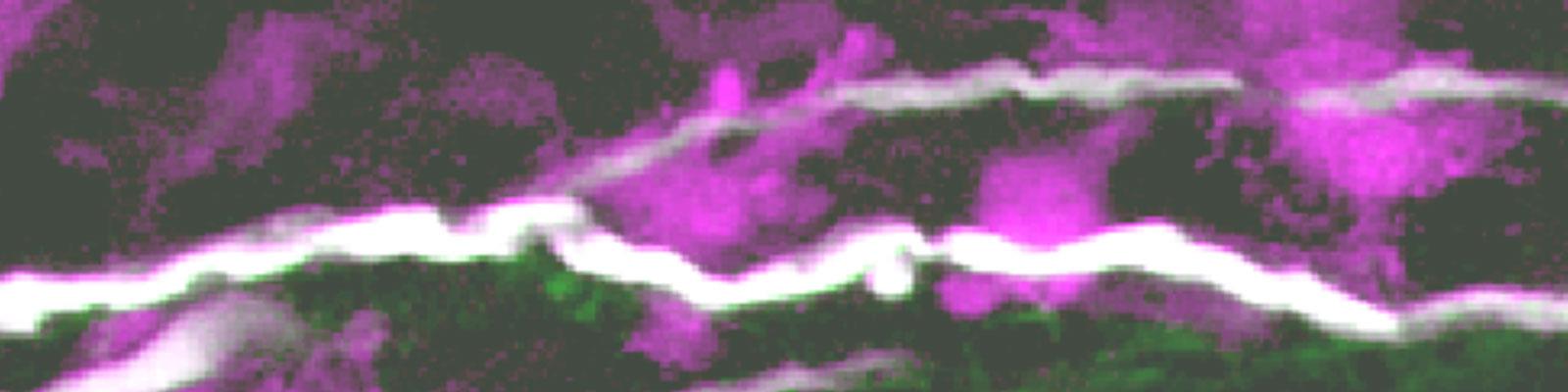
In vivo pathogenesis of immune mediated axon damage
We want to understand how immune cells damage axons in neuroinflammatory lesions. We have identified Focal Axonal Degeneration as a cellular mechanism of immune-mediated axon degeneration in both experimental and human neuroinflammatory lesions. As the early stages of this process are reversible we are now trying to unravel which cellular processes and molecular signals drive axons towards fragmentation or allow them to recover.
Project-related publications
Tai YH, Engels D, Locatelli G, Emmanouilidis I, Fecher C, Theodorou D, Müller SA, Licht-Mayer S, Kreutzfeldt M, Wagner I, de Mello NP, Gkotzamani SN, Trovò L, Kendirli A, Aljović A, Breckwoldt MO, Naumann R, Bareyre FM, Perocchi F, Mahad D, Merkler D, Lichtenthaler SF, Kerschensteiner M, Misgeld T. (2023). Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions. Nature metabolism, 5(8), 1364–1381.
Witte ME, Schumacher AM, Mahler CF, Bewersdorf JP, Lehmitz J, Scheiter A, Sánchez P, Williams PR, Griesbeck O, Naumann R, Misgeld T, Kerschensteiner M. Calcium Influx through Plasma-Membrane Nanoruptures Drives Axon Degeneration in a Model of Multiple Sclerosis. Neuron 2019 Feb 20;101(4):615-624
Sorbara CD, Wagner NE, Ladwig A, Nikić I, Merkler D, Kleele T, Marinković P, Naumann R, Godinho L, Bareyre FM, Bishop D, Misgeld T, Kerschensteiner M. Pervasive axonal transport deficits in multiple sclerosis models. Neuron 2014 Dec 17;84(6):1183-90.
Nikić I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, Brück W, Bishop D, Misgeld T, Kerschensteiner M. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2011 Apr;17(4):495-9.
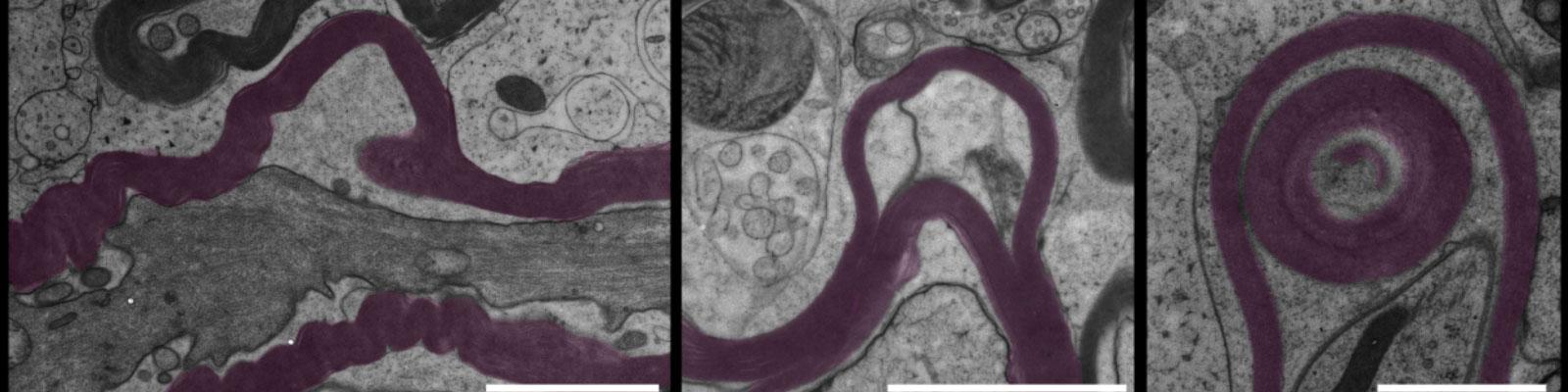
Mechanisms of oligodendrocyte damage and recovery
We are interested in resolving where and how oligodendrocytes are attacked by immune cells. Our work shows that damage often follows an outside-in pattern resulting in amputated oligodendrocytes. We now want to understand the fate of these cells during the formation and recovery of demyelinating lesions.
Project-related publications
Mezydlo A, Treiber N, Ullrich Gavilanes EM, Eichenseer K, Ancău M, Wens A, Ares Carral C, Schifferer M, Snaidero N, Misgeld T, Kerschensteiner M. Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron. 2023 Apr 13:S0896-6273(23)00227-1.
Snaidero N, Schifferer M, Mezydlo A, Zalc B, Kerschensteiner M, Misgeld T. Myelin replacement triggered by single-cell demyelination in mouse cortex. Nat Commun. 2020 Sep 29;11(1):4901.
Romanelli E, Merkler D, Mezydlo A, Weil MT, Weber MS, Nikić I, Potz S, Meinl E, Matznick FE, Kreutzfeldt M, Ghanem A, Conzelmann KK, Metz I, Brück W, Routh M, Simons M, Bishop D, Misgeld T, Kerschensteiner M. Myelinosome formation represents an early stage of oligodendrocyte damage in multiple sclerosis and its animal model. Nat Commun. 2016 Nov 16;7:13275.

Regulation of phagocyte responses in neuroinflammatory lesions
Mononuclear phagocytes such as macrophages and microglial cells can contribute both to the formation and resolution of neuroinflammatory lesions. They do so because they shift their molecular characteristics and functional properties as lesions evolve. Here we want to dissect the immune cell- and nervous system-derived signaling streams that govern phagocyte phenotypes in the inflamed CNS.
Project-related publications
Jordão MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Tai YH, Tay TL, Schramm E, Armbruster S, Hagemeyer N, Groß O, Mai D, Çiçek Ö, Falk T, Kerschensteiner M, Grün D, Prinz M. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 2019 Jan 25;363(6425):eaat7554
Locatelli G, Theodorou D, Kendirli A, Jordão MJC, Staszewski O, Phulphagar K, Cantuti-Castelvetri L, Dagkalis A, Bessis A, Simons M, Meissner F, Prinz M, Kerschensteiner M. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat Neurosci. 2018 Sep;21(9):1196-1208.
Goldmann T, Wieghofer P, Jordão MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FM, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016 Jul;17(7):797-805.

Models, markers and manipulation of inflammatory gray matter pathology
While white matter lesions dominate the early stages of multiple sclerosis, the gray matter takes center stage as the disease advances. We thus try to better characterize gray matter pathology in multiple sclerosis patients, develop models that allow us to address distinct aspects of gray matter pathology and thereby aim to resolve pathological targets, biological markers and emerging therapies for MS progression.
Project-related publications
Jafari M, Schumacher A-M, Snaidero N, Neziraj T, Ullrich Gavilianes EM, Jürgens T, Florez Weidinger JD, Schmidt SS, Beltran E, Hagan N, Woodworth L, Ofengeim D, Gans J, Wolf F, Kreutzfeldt M, Portugues R, Merkler D, Misgeld T, Kerschensteiner M. Localized calcium accumulations prime synapses for phagocyte removal in cortical neuroinflammation. Nat Neurosci. 2021 Mar;24(3):355-367.
Jürgens T, Jafari M, Kreutzfeldt M, Bahn E, Brück W, Kerschensteiner M, Merkler D. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain 2016 Jan;139(Pt 1):39-46.

Fluorogenic chemical probes for wash-free imaging of cell membrane damage in ferroptosis, necrosis and axon injury
A genome-wide in vivo CRISPR screen identifies essential regulators of T cell migration to the CNS in a multiple sclerosis model.

October 2023 –The infiltration of autoreactive T cells to the central nervous system (CNS) triggers lesion formation in the common neuroinflammatory condition multiple sclerosis (MS). Here, the Kawakami and Kerschensteiner labs worked together to provide a comprehensive characterization of the essential molecules that regulate this transmigration process. By performing a genome-wide CRISPR screen in a rat MS model they could identify 18 essential facilitators of T cell migration to the CNS. These can be grouped into three functional modules that regulate T cell adhesion, chemotaxis and egress. Notably, many of these molecules show regulated expression in T cell from MS patients and include prominent targets of approved MS therapies.
Kendirli A, de la Rosa C, Lämmle KF, Eglseer K, Bauer IJ, Kavaka V, Winklmeier S, Zhuo L, Wichmann C, Gerdes LA, Kümpfel T, Dornmair K, Beltrán E, Kerschensteiner M*, Kawakami N* (2023) A genome-wide in vivo CRISPR screen identifies essential regulators of T cell migration to the CNS in a multiple sclerosis model. Nat Neurosci. 26(10):1713-1725.
Press release : https://www.lmu.de/en/newsroom/news-overview/news/identified-key-regulators-involved-in-genesis-of-multiple-sclerosis-lesions.html
Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions.

August 2023 – Inflammation in the central nervous system can impair the function of neuronal mitochondria, the energy-producing components within nerve cells, which contributes to axonal degeneration in multiple sclerosis (MS). Here the Kerschensteiner lab at LMU Munich and the Misgeld lab at TU Munich have teamed up to unravel how inflammation changes the molecular composition and functional capacity of neuronal mitochondria. This work identifies the TCA cycle as new potential target for therapeutic interventions to protect nerve cells in MS.
Tai YH, Engels D, Locatelli G, Emmanouilidis I, Fecher C, Theodorou D, Müller SA, Licht-Mayer S, Kreutzfeldt M, Wagner I, de Mello NP, Gkotzamani SN, Trovò L, Kendirli A, Aljović A, Breckwoldt MO, Naumann R, Bareyre FM, Perocchi F, Mahad D, Merkler D, Lichtenthaler SF, Kerschensteiner M, Misgeld T. (2023). Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions. Nature metabolism, 5(8), 1364–1381.
Press release Synergy: https://www.synergy-munich.de/news/news/kerschensteiner-misgeld/index.html
Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex.

April 2023 – In multiple sclerosis immune-mediated myelin loss can be partially reversed by remyelination. Here the Kerschensteiner lab and the Misgeld lab at TUM investigate if and how mature oligodendrocytes that survive in the lesion area in both MS patients and models can contribute to the myelin repair process. In a mouse model of cortical MS pathology they show that while surviving oligodendrocytes can extend new proximal processes they rarely form new myelin internodes. Furthermore drugs that boost myelin recovery by targeting oligodendrocyte precursor cells did not enhance myelin repair by surviving oligodendrocytes indicating that distinct therapeutic approaches are required to recruit surviving oligodendrocytes to the remyelination process.
Mezydlo A, Treiber N, Ullrich Gavilanes EM, Eichenseer K, Ancău M, Wens A, Ares Carral C, Schifferer M, Snaidero N, Misgeld T, Kerschensteiner M. Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron. 2023 Apr 13:S0896-6273(23)00227-1.
PET imaging can improve montoring of Natalizumab-associated PML

November 2021 – Progressive multifocal leukoencephalopathy (PML) is a severe CNS infection that can occur in MS patients treated with Natalizumab. Clinical management of patients with Natalizumab-associated PML is challenging not least to detect, monitor and differentiate PML lesions are limited. Here the Kerschensteiner lab and Kümpfel group teamed up to investigate whether TSPO PET imaging can be applied to monitor the inflammatory activity of PML lesions over time. The results of this monocentre pilot study now indicate that TSPO PET imaging may faciliate longitudinal monitoring of disease activity and help distinguish recurrent multiple sclerosis activity from PML progression.
Mahler C, Schumacher AM, Unterrainer M, Kaiser L, Höllbacher T, Lindner S, Havla J, Ertl-Wagner B, Patzig M, Seelos K, Neitzel J, Mäurer M, Krumbholz M, Metz I, Brück W, Stadelmann C, Merkler D, Gass A, Milenkovic V, Bartenstein P, Albert NL, Kümpfel T, Kerschensteiner M. TSPO PET imaging of natalizumab-associated progressive multifocal leukoencephalopathy. Brain. 2021 Oct 22;144(9):2683-2695.
Immune cells remove synapses in the inflamed gray matter

January 2021 – Gray matter pathology is a critical contributor to disability in advanced stages of multiple sclerosis. Here the Kerschensteiner lab teamed up with the Misgeld and Merkler lab to investigate how neuronal structure and function is impacted in the neuronal gray matter. In a mouse model they show that gray matter inflammation leads to a synapse loss that is accompagnied by neuronal silencing and can be reversible. Synapse loss is primed by local calcium increases, executed by activated microglial cells and infiltrating monocyte-derived macrophages and can be mitigated by therapeutic strategies that interfer with pathological activation of phagocytes. The authors hope that their work can help the development of therapeutic approaches that curb progression in MS patients.
Jafari M, Schumacher AM, Snaidero N, Ullrich Gavilanes EM, Neziraj T, Kocsis-Jutka V, Engels D, Jürgens T, Wagner I, Weidinger JDF, Schmidt SS, Beltrán E, Hagan N, Woodworth L, Ofengeim D, Gans J, Wolf F, Kreutzfeldt M, Portugues R, Merkler D, Misgeld T, Kerschensteiner M. Phagocyte-mediated synapse removal in cortical neuroinflammation is promoted by local calcium accumulation. Nat Neurosci. 2021 Mar;24(3):355-367.
Single cell ablation reveals the rules of myelin replacement

September 2020 – To interrogate the rules that govern myelin replacement in the cortex the Kerschensteiner group together with the Misgeld lab at TUM studied the response to the ablation of single cortical oligodendrocytes. Using timelapse imaging and correlated ultrastructural reconstructions they were able to show that a loss of a single oligodendrocyte is sufficient to cause robust cell and myelin replacement. In this process internodes along partially myelinated axons were typically not reestablished, while myelin sheaths forming continuous patterns showed remarkable homeostatic resiliences and remyelinated with single axon precision. Understanding these principles is particularly important in the context of multiple sclerosis which extensive cortical demyelination is a major pathological feature and target of remyelination therapies.
Snaidero N, Schifferer M, Mezydlo A, Zalc B, Kerschensteiner M, Misgeld T. Myelin replacement triggered by single-cell demyelination in mouse cortex. Nat Commun. 2020 Sep 29;11(1):4901.
Leaky membranes promote axon degeneration in MS model

Februar 2019 – To reveal the mechanisms that drive inflammatory axon degeneration the Kerschensteiner lab has used in vivo calcium imaging in a multiple sclerosis model. Dynamic tracking of individual axons in the inflamed spinal cord shows that cytoplasmic calcium levels determine the choice between axon loss and survival. Calcium can enter the axon through nanoscale ruptures of the axonal plasma membrane that are induced in inflammatory lesions. These results thus identify a unusual axon injury pathway that can contribute to neurodegeneration in multiple sclerosis and may represent a novel target for protective therapy.
Witte ME, Schumacher AM, Mahler CF, Bewersdorf JP, Lehmitz J, Scheiter A, Sánchez P, Williams PR, Griesbeck O, Naumann R, Misgeld T, Kerschensteiner M. Calcium Influx through Plasma-Membrane Nanoruptures Drives Axon Degeneration in a Model of Multiple Sclerosis. Neuron. 2019 Feb 20;101(4):615-624.e5.
Shifty phagocytes track the fate of neuroinflammatory lesions

Februar 2018 – Mononuclear phagocytes can both promote and inhibit inflammation. Here the Kerschensteiner lab uses an in vivo imaging approach to follow the evolution of phagocyte phenotypes in neuroinflammatory lesions. By tracking phagocytes over time they can show that individual phagocytes switch their phenotype as lesions move from expansion to resolution. This phenotype shift appears to be initiated by signals derived from the nervous system tissue. Understanding the molecular nature of these signals might thus provide the basis for therapeutic manipulation of phagocyte function in the inflamed CNS.
Locatelli G, Theodorou D, Kendirli A, Jordão MJC, Staszewski O, Phulphagar K, Cantuti-Castelvetri L, Dagkalis A, Bessis A, Simons M, Meissner F, Prinz M, Kerschensteiner M. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat Neurosci. 2018 Sep;21(9):1196-1208.
Early stages of myelin injury revealed by ultrastructural and dynamic analysis

November 2016 – Damage to oligodendrocytes and their myelin sheaths is a central feature of multiple sclerosis pathology. Here the Kerschensteiner lab investigates how oligodendrocyte damage is initiated in multiple sclerosis (MS) and EAE using in vivo imaging, confocal microscopy as well as electron microscopy. They can show that oligodendrocyte damage spreads centripetally and that the formation of focal myelin outfoldings that they call “myelinosomes” is an early sign of oligodendrocyte damage both in MS and EAE…
Romanelli E, Merkler D, Mezydlo A, Weil MT, Weber MS, Nikić I, Potz S, Meinl E, Matznick FE, Kreutzfeldt M, Ghanem A, Conzelmann KK, Metz I, Brück W, Routh M, Simons M, Bishop D, Misgeld T, Kerschensteiner M. Myelinosome formation represents an early stage of oligodendrocyte damage in multiple sclerosis and its animal model. Nat Commun. 2016 Nov 16;7:13275.
Widespread synapse loss in multiple sclerosis gray matter

January 2016 – How neurodegeneration starts in the brains of progressive multiple sclerosis patients is only incompletely understood. Here the Kerschensteiner lab joined forces with the Merkler lab at the University of Genevan and used confocal microscopy of Golgi-Cox impregnated tissue sections to reconstruct single cortical projection neurons in brain sections from multiple sclerosis and control patients. Their study reveals a widespread and pronounced loss of dendritic spines that occurs independently of cortical demyelination and axon loss and indicates the presence of a primary synaptic pathology in multiple sclerosis…
Jürgens T, Jafari M, Kreutzfeldt M, Bahn E, Brück W, Kerschensteiner M, Merkler D. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain. 2016 Jan;139(Pt 1):39-46.
Window of opportunity for axonal rescue after spinal cord contusion

December 2014 – The loss of axonal connectivity underlies the devastating consequences of spinal cord injuries. Here the Misgeld lab at TUM and the Kerschensteiner lab use spinal in vivo imaging to show that axons persist in a metastable state for hours after spinal contusion injury. During this time the intra-axonal calcium dynamics determine the axonal fate. Those axons that manage to re-establish calcium homoestasis will survive ong term while those that fail to do so will degenerate. This study identifies an inherent self-preseravtion process in contused axons and a window of opportunity for rescuing axonal connectivity after contusion injury.
Williams PR, Marincu BN, Sorbara CD, Mahler CF, Schumacher AM, Griesbeck O, Kerschensteiner M, Misgeld T. A recoverable state of axon injury persists for hours after spinal cord contusion in vivo. Nat Commun. 2014 Dec 16;5:5683.
Axonal traffic jams in neuroinflammatory lesions

December 2014 – The axonal transport of cargos from the neuronal cell body to the synapse (and back) is key for maintaining neuronal health and function. Here the Kerschensteiner lab visualizes the dynamics of organelles in individual spinal axons and reveals widespread impairment of organelle transport in MS models. Transport deficits precede structural alterations of axons, myelin or microtubules. They persist in chronic MS models and lead to a reduced distal organelle density – a possible first step towards axonal degeneration.
Sorbara CD, Wagner NE, Ladwig A, Nikić I, Merkler D, Kleele T, Marinković P, Naumann R, Godinho L, Bareyre FM, Bishop D, Misgeld T, Kerschensteiner M. Pervasive axonal transport deficits in multiple sclerosis models. Neuron. 2014 Dec 17;84(6):1183-90.
A novel multiparametric imaging approach to study mitochondria

May 2014 – Mitochondria the main energy source of the cell play a key role in neuronal physiology and pathology. Here the Misgeld (TUM) and Kerschensteiner (LMU) labs establish a novel multiparametric imaging approach that allows to visualize and mechanistically dissect mitochondrial redox signals with high temporal and spatial resolution in the living nervous system. This new technique thus offers new dynamic insights into mitochondrial signaling in the healthy or diseased nervous system.
Breckwoldt MO, Pfister FM, Bradley PM, Marinković P, Williams PR, Brill MS, Plomer B, Schmalz A, St Clair DK, Naumann R, Griesbeck O, Schwarzländer M, Godinho L, Bareyre FM, Dick TP, Kerschensteiner M, Misgeld T. Multiparametric optical analysis of mitochondrial redox signals during neuronal physiology and pathology in vivo. Nat Med. 2014 May;20(5):555-60.
2024
Gross, C. C., Schulte-Mecklenbeck, A., Steinberg, O. V., Wirth, T., Lauks, S., Bittner, S., Schindler, P., Baranzini, S. E., Groppa, S., Bellmann-Strobl, J., Bünger, N., Chien, C., Dawin, E., Eveslage, M., Fleischer, V., Gonzalez-Escamilla, G., Gisevius, B., Haas, J., Kerschensteiner, M., Kirstein, L., … German Competence Network Multiple Sclerosis (KKNMS) (2024). Multiple sclerosis endophenotypes identified by high-dimensional blood signatures are associated with distinct disease trajectories. Science translational medicine, 16(740), eade8560.
Mauker, P., Beckmann, D., Kitowski, A., Heise, C., Wientjens, C., Davidson, A. J., Wanderoy, S., Fabre, G., Harbauer, A. B., Wood, W., Wilhelm, C., Thorn-Seshold, J., Misgeld, T., Kerschensteiner, M., & Thorn-Seshold, O. (2024). Fluorogenic Chemical Probes for Wash-free Imaging of Cell Membrane Damage in Ferroptosis, Necrosis, and Axon Injury. Journal of the American Chemical Society, 10.1021/jacs.3c07662. Advance online publication.
2023
Kolabas ZI, Kuemmerle LB, Perneczky R, Förstera B, Ulukaya S, Ali M, Kapoor S, Bartos LM, Büttner M, Caliskan OS, Rong Z, Mai H, Höher L, Jeridi D, Molbay M, Khalin I, Deligiannis IK, Negwer M, Roberts K, Simats A, Carofiglio O, Todorov MI, Horvath I, Ozturk F, Hummel S, Biechele G, Zatcepin A, Unterrainer M, Gnörich J, Roodselaar J, Shrouder J, Khosravani P, Tast B, Richter L, Díaz-Marugán L, Kaltenecker D, Lux L, Chen Y, Zhao S, Rauchmann BS, Sterr M, Kunze I, Stanic K, Kan VWY, Besson-Girard S, Katzdobler S, Palleis C, Schädler J, Paetzold JC, Liebscher S, Hauser AE, Gokce O, Lickert H, Steinke H, Benakis C, Braun C, Martinez-Jimenez CP, Buerger K, Albert NL, Höglinger G, Levin J, Haass C, Kopczak A, Dichgans M, Havla J, Kümpfel T, Kerschensteiner M, Schifferer M, Simons M, Liesz A, Krahmer N, Bayraktar OA, Franzmeier N, Plesnila N, Erener S, Puelles VG, Delbridge C, Bhatia HS, Hellal F, Elsner M, Bechmann I, Ondruschka B, Brendel M, Theis FJ, Erturk A. (2023) Distinct molecular profiles of skull bone marrow in health and neurological disorders. Cell. 186(17):3706-3725.e29.
Ballweg A, Klaus C, Vogler L, Katzdobler S, Wind K, Zatcepin A, Ziegler SI, Secgin B, Eckenweber F, Bohr B, Bernhardt A, Fietzek U, Rauchmann BS, Stoecklein S, Quach S, Beyer L, Scheifele M, Simmet M, Joseph E, Lindner S, Berg I, Koglin N, Mueller A, Stephens AW, Bartenstein P, Tonn JC, Albert NL, Kümpfel T, Kerschensteiner M, Perneczky R, Levin J, Paeger L, Herms J, Brendel M.(2023) [18F]F-DED PET imaging of reactive astrogliosis in neurodegenerative diseases: preclinical proof of concept and first-in-human data. J Neuroinflammation. 20(1):68.
Kislinger G, Niemann C, Rodriguez L, Jiang H, Fard MK, Snaidero N, Schumacher AM, Kerschensteiner M, Misgeld T, Schifferer M. (2023) Neurons on tape: Automated Tape Collecting Ultramicrotomy-mediated volume EM for targeting neuropathology. Methods Cell Biol. 177:125-170.
Kendirli A, de la Rosa C, Lämmle KF, Eglseer K, Bauer IJ, Kavaka V, Winklmeier S, Zhuo L, Wichmann C, Gerdes LA, Kümpfel T, Dornmair K, Beltrán E, Kerschensteiner M*, Kawakami N* (2023) A genome-wide in vivo CRISPR screen identifies essential regulators of T cell migration to the CNS in a multiple sclerosis model. Nat Neurosci. 26(10):1713-1725.
Tai, Y. H., Engels, D., Locatelli, G., Emmanouilidis, I., Fecher, C., Theodorou, D., Müller, S. A., Licht-Mayer, S., Kreutzfeldt, M., Wagner, I., de Mello, N. P., Gkotzamani, S. N., Trovò, L., Kendirli, A., Aljović, A., Breckwoldt, M. O., Naumann, R., Bareyre, F. M., Perocchi, F., Mahad, D., … Misgeld, T. (2023). Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions. Nature metabolism, 5(8), 1364–1381.
Mezydlo A, Treiber N, Ullrich Gavilanes EM, Eichenseer K, Ancău M, Wens A, Ares Carral C, Schifferer M, Snaidero N, Misgeld T, Kerschensteiner M. Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron. 2023 Apr 13:S0896-6273(23)00227-1.
Biljecki M, Eisenhut K, Beltrán E, Winklmeier S, Mader S, Thaller A, Eichhorn P, Steininger P, Flierl-Hecht A, Lewerenz J, Kümpfel T, Kerschensteiner M, Meinl E, Thaler FS. Antibodies Against Glutamic Acid Decarboxylase 65 Are Locally Produced in the CSF and Arise During Affinity Maturation. Neurol Neuroimmunol Neuroinflamm. 2023 Feb 23;10(3):e200090.
Aljović A, Jacobi A, Marcantoni M, Kagerer F, Loy K, Kendirli A, Bräutigam J, Fabbio L, Van Steenbergen V, Pleśniar K, Kerschensteiner M, Bareyre FM. Synaptogenic gene therapy with FGF22 improves circuit plasticity and functional recovery following spinal cord injury. EMBO Mol Med. 2023 Jan 5:e16111.
2022
Schneider-Hohendorf T, Gerdes LA, Pignolet B, Gittelman R, Ostkamp P, Rubelt F, Raposo C, Tackenberg B, Riepenhausen M, Janoschka C, Wünsch C, Bucciarelli F, Flierl-Hecht A, Beltrán E, Kümpfel T, Anslinger K, Gross CC, Chapman H, Kaplan I, Brassat D, Wekerle H, Kerschensteiner M, Klotz L, Lünemann JD, Hohlfeld R, Liblau R, Wiendl H, Schwab N. Broader Epstein-Barr virus-specific T cell receptor repertoire in patients with multiple sclerosis. J Exp Med. 2022 Nov 7;219(11):e20220650.
Unterrainer M, Mahler C, Schumacher AM, Ruf V, Blum B, Quach S, Brendel M, Rupprecht R, Bartenstein P, Kerschensteiner M, Kümpfel T, Albert NL. Amino Acid Uptake, Glucose Metabolism, and Neuroinflammation in John Cunningham Virus Associated Progressive Multifocal Leukoencephalopathy. Clin Nucl Med. 2022 Jun 1;47(6):543-544.
Ingelfinger F, Gerdes LA, Kavaka V, Krishnarajah S, Friebel E, Galli E, Zwicky P, Furrer R, Peukert C, Dutertre CA, Eglseer KM, Ginhoux F, Flierl-Hecht A, Kümpfel T, De Feo D, Schreiner B, Mundt S, Kerschensteiner M, Hohlfeld R, Beltrán E, Becher B. Twin study reveals non-heritable immune perturbations in multiple sclerosis. Nature. 2022 Mar;603(7899):152-158.
Kerschensteiner M, Misgeld T. A less painful transfer of power. Neuron. 2022 Feb 16;110(4):559-561.
Aljovic A, Zhao S, Chahin M, de la Rosa C, Van Steenbergen V, Kerschensteiner M, Bareyre FM. A deep learning-based toolbox for Automated Limb Motion Analysis (ALMA) in murine models of neurological disorders. Commun Biol. 2022 Feb 15;5(1):131.
2021
Schifferer M, Snaidero N, Djannatian M, Kerschensteiner M, Misgeld T. Niwaki Instead of Random Forests: Targeted Serial Sectioning Scanning Electron Microscopy With Reimaging Capabilities for Exploring Central Nervous System Cell Biology and Pathology. Front Neuroanat. 2021 Oct 13;15:732506.
Jafari M, Schumacher AM, Snaidero N, Ullrich Gavilanes EM, Neziraj T, Kocsis-Jutka V, Engels D, Jürgens T, Wagner I, Weidinger JDF, Schmidt SS, Beltrán E, Hagan N, Woodworth L, Ofengeim D, Gans J, Wolf F, Kreutzfeldt M, Portugues R, Merkler D, Misgeld T, Kerschensteiner M. Phagocyte-mediated synapse removal in cortical neuroinflammation is promoted by local calcium accumulation. Nat Neurosci. 2021 Mar;24(3):355-367.
Mahler C, Schumacher AM, Unterrainer M, Kaiser L, Höllbacher T, Lindner S, Havla J, Ertl-Wagner B, Patzig M, Seelos K, Neitzel J, Mäurer M, Krumbholz M, Metz I, Brück W, Stadelmann C, Merkler D, Gass A, Milenkovic V, Bartenstein P, Albert NL, Kümpfel T, Kerschensteiner M. TSPO PET imaging of natalizumab-associated progressive multifocal leukoencephalopathy. Brain. 2021 Oct 22;144(9):2683-2695.
Penkert H, Lauber C, Gerl MJ, Klose C, Damm M, Fitzner D, Flierl-Hecht A, Kümpfel T, Kerschensteiner M, Hohlfeld R, Gerdes LA, Simons M. Plasma lipidomics of monozygotic twins discordant for multiple sclerosis. Ann Clin Transl Neurol. 2020 Dec;7(12):2461-2466.
2020
Snaidero N, Schifferer M, Mezydlo A, Zalc B, Kerschensteiner M*, Misgeld T. Myelin replacement triggered by single-cell demyelination in mouse cortex. Nat Commun. 2020 Sep 29;11(1):4901.(* co-senior author)
Kislinger G, Gnägi H, Kerschensteiner M, Simons M, Misgeld T, Schifferer M. ATUM-FIB microscopy for targeting and multiscale imaging of rare events in mouse cortex. STAR Protoc. 2020 Dec 16;1(3):100232.
Kislinger G, Gnägi H, Kerschensteiner M, Simons M, Misgeld T, Schifferer M. Multiscale ATUM-FIB Microscopy Enables Targeted Ultrastructural Analysis at Isotropic Resolution. iScience. 2020 Jul 24;23(7):101290.
Sauerbeck AD, Gangolli M, Reitz SJ, Salyards MH, Kim SH, Hemingway C, Gratuze M, Makkapati T, Kerschensteiner M, Holtzman DM, Brody DL, Kummer TT. SEQUIN Multiscale Imaging of Mammalian Central Synapses Reveals Loss of Synaptic Connectivity Resulting from Diffuse Traumatic Brain Injury. Neuron. 2020 Jul 22;107(2):257-273.e5.
2019
Bradley PM, Denecke CK, Aljovic A, Schmalz A, Kerschensteiner M, Bareyre FM. Corticospinal circuit remodeling after central nervous system injury is dependent on neuronal activity. J Exp Med. 2019 Nov 4;216(11):2503-2514..
Schumacher AM, Misgeld T, Kerschensteiner M, Snaidero N. Imaging the execution phase of neuroinflammatory disease models. Exp Neurol. 2019 Oct;320:112968.
Eisele P, Konstandin S, Szabo K, Ebert A, Roßmanith C, Paschke N, Kerschensteiner M, Platten M, Schoenberg SO, Schad LR, Gass A. Temporal evolution of acute multiple sclerosis lesions on serial sodium (23Na) MRI. Mult Scler Relat Disord. 2019 Apr;29:48-54
Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Förstera B, Zhao S, Bhatia HS, Parra-Damas A, Mrowka L, Theodorou D, Rempfler M, Xavier ALR, Kress BT, Benakis C, Steinke H, Liebscher S, Bechmann I, Liesz A, Menze B, Kerschensteiner M, Nedergaard M, Ertürk A. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat Neurosci. 2019 Feb;22(2):317-327.
Witte ME, Schumacher AM, Mahler CF, Bewersdorf JP, Lehmitz J, Scheiter A, Sánchez P, Williams PR, Griesbeck O, Naumann R, Misgeld T, Kerschensteiner M. Calcium Influx through Plasma-Membrane Nanoruptures Drives Axon Degeneration in a Model of Multiple Sclerosis. Neuron. 2019 Feb 20;101(4):615-624
Jordão MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Tai YH, Tay TL, Schramm E, Armbruster S, Hagemeyer N, Groß O, Mai D, Çiçek Ö, Falk T, Kerschensteiner M, Grün D, Prinz M. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019 Jan 25;363(6425):eaat7554.
2018
Locatelli G, Theodorou D, Kendirli A, Jordão MJC, Staszewski O, Phulphagar K, Cantuti-Castelvetri L, Dagkalis A, Bessis A, Simons M, Meissner F, Prinz M, Kerschensteiner M. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat Neurosci. 2018 Sep;21(9):1196-1208.
Unterrainer M, Mahler C, Vomacka L, Lindner S, Havla J, Brendel M, Böning G, Ertl-Wagner B, Kümpfel T, Milenkovic VM, Rupprecht R, Kerschensteiner M, Bartenstein P, Albert NL. TSPO PET with [18F]GE-180 sensitively detects focal neuroinflammation in patients with relapsing-remitting multiple sclerosis. Eur J Nucl Med Mol Imaging. 2018 Jul;45(8):1423-1431.
2017
Vomacka L, Albert NL, Lindner S, Unterrainer M, Mahler C, Brendel M, Ermoschkin L, Gosewisch A, Brunegraf A, Buckley C, Kümpfel T, Rupprecht R, Ziegler S, Kerschensteiner M, Bartenstein P, Böning G. TSPO imaging using the novel PET ligand [18F]GE-180: quantification approaches in patients with multiple sclerosis. EJNMMI Res. 2017 Oct 26;7(1):89.
Mazaheri F, Snaidero N, Kleinberger G, Madore C, Daria A, Werner G, Krasemann S, Capell A, Trümbach D, Wurst W, Brunner B, Bultmann S, Tahirovic S, Kerschensteiner M, Misgeld T, Butovsky O, Haass C. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. EMBO Rep. 2017 Jul;18(7):1186-1198.
2016
Romanelli E, Merkler D, Mezydlo A, Weil MT, Weber MS, Nikić I, Potz S, Meinl E, Matznick FE, Kreutzfeldt M, Ghanem A, Conzelmann KK, Metz I, Brück W, Routh M, Simons M, Bishop D, Misgeld T, Kerschensteiner M. Myelinosome formation represents an early stage of oligodendrocyte damage in multiple sclerosis and its animal model. Nat Commun. 2016 Nov 16;7:13275.
Hohlfeld R, Kerschensteiner M. Antiglutamatergic therapy for multiple sclerosis? Lancet Neurol. 2016 Sep;15(10):1003-4.
Weil MT, Möbius W, Winkler A, Ruhwedel T, Wrzos C, Romanelli E, Bennett JL, Enz L, Goebels N, Nave KA, Kerschensteiner M, Schaeren-Wiemers N, Stadelmann C, Simons M. Loss of Myelin Basic Protein Function Triggers Myelin Breakdown in Models of Demyelinating Diseases. Cell Rep. 2016 Jul 12;16(2):314-322.
Goldmann T, Wieghofer P, Jordão MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FM, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016 Jul;17(7):797-80
Fujikawa Y, Roma LP, Sobotta MC, Rose AJ, Diaz MB, Locatelli G, Breckwoldt MO, Misgeld T, Kerschensteiner M, Herzig S, Müller-Decker K, Dick TP. Mouse redox histology using genetically encoded probes. Sci Signal. 2016 Mar 15;9(419):rs1
Jürgens T, Jafari M, Kreutzfeldt M, Bahn E, Brück W, Kerschensteiner M*, Merkler D. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain. 2016 Jan;139(Pt 1):39-46. (* co-senior author)
2015
Schreiner B, Romanelli E, Liberski P, Ingold-Heppner B, Sobottka-Brillout B, Hartwig T, Chandrasekar V, Johannssen H, Zeilhofer HU, Aguzzi A, Heppner F, Kerschensteiner M, Becher B. Astrocyte Depletion Impairs Redox Homeostasis and Triggers Neuronal Loss in the Adult CNS. Cell Rep. 2015 Sep 1;12(9):1377-8
Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015 Apr;14(4):406-19.
Jacobi A, Loy K, Schmalz AM, Hellsten M, Umemori H, Kerschensteiner M, Bareyre FM. FGF22 signaling regulates synapse formation during post-injury remodeling of the spinal cord. EMBO J. 2015 May 5;34(9):1231-43.
Breckwoldt MO, Wittmann C, Misgeld T, Kerschensteiner M, Grabher C. Redox imaging using genetically encoded redox indicators in zebrafish and mice. Biol Chem. 2015 May;396(5):511-22.
2014
Williams PR, Marincu BN, Sorbara CD, Mahler CF, Schumacher AM, Griesbeck O, Kerschensteiner M, Misgeld T. A recoverable state of axon injury persists for hours after spinal cord contusion in vivo. Nat Commun. 2014 Dec 16;5:5683
Sorbara CD, Wagner NE, Ladwig A, Nikić I, Merkler D, Kleele T, Marinković P, Naumann R, Godinho L, Bareyre FM, Bishop D, Misgeld T, Kerschensteiner M. Pervasive axonal transport deficits in multiple sclerosis models. Neuron. 2014 Dec 17;84(6):1183-90.
Bishop D, Nikic I, Kerschensteiner M, Misgeld T. The use of a laser for correlating light and electron microscopic images in thick tissue specimens. Methods Cell Biol. 2014;124:323-37.
Kleele T, Marinković P, Williams PR, Stern S, Weigand EE, Engerer P, Naumann R, Hartmann J, Karl RM, Bradke F, Bishop D, Herms J, Konnerth A, Kerschensteiner M, Godinho L, Misgeld T. An assay to image neuronal microtubule dynamics in mice. Nat Commun. 2014 Sep 12;5:4827.
Simons M, Misgeld T, Kerschensteiner M. A unified cell biological perspective on axon-myelin injury. J Cell Biol. 2014 Aug 4;206(3):335-45.
Breckwoldt MO, Pfister FM, Bradley PM, Marinković P, Williams PR, Brill MS, Plomer B, Schmalz A, St Clair DK, Naumann R, Griesbeck O, Schwarzländer M, Godinho L, Bareyre FM, Dick TP, Kerschensteiner M*, Misgeld T. Multiparametric optical analysis of mitochondrial redox signals during neuronal physiology and pathology in vivo. Nat Med. 2014 May;20(5):555-60. (* co-senior author)
2013
Kreutzfeldt M, Bergthaler A, Fernandez M, Brück W, Steinbach K, Vorm M, Coras R, Blümcke I, Bonilla WV, Fleige A, Forman R, Müller W, Becher B, Misgeld T, Kerschensteiner M, Pinschewer DD, Merkler D. Neuroprotective intervention by interferon-γ blockade prevents CD8+ T cell-mediated dendrite and synapse loss. J Exp Med. 2013 Sep 23;210(10):2087-103.
Lang C, Bradley PM, Jacobi A, Kerschensteiner M, Bareyre FM. STAT3 promotes corticospinal remodelling and functional recovery after spinal cord injury. EMBO Rep. 2013 Oct;14(10):931-7.
Romanelli E, Sorbara CD, Nikić I, Dagkalis A, Misgeld T, Kerschensteiner M. Cellular, subcellular and functional in vivo labeling of the spinal cord using vital dyes. Nat Protoc. 2013 Mar;8(3):481-90.
2012
Sorbara C, Misgeld T, Kerschensteiner M. In vivo imaging of the diseased nervous system: an update. Curr Pharm Des. 2012;18(29):4465-70.
Marinkovic P, Reuter MS, Brill MS, Godinho L, Kerschensteiner M*, Misgeld T. Axonal transport deficits and degeneration can evolve independently in mouse models of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2012 Mar 13;109(11):4296-301. (* co-senior author)
Lang C, Guo X, Kerschensteiner M, Bareyre FM. Single collateral reconstructions reveal distinct phases of corticospinal remodeling after spinal cord injury. PLoS One. 2012;7(1):e30461.
2011
Bishop D, Nikić I, Brinkoetter M, Knecht S, Potz S, Kerschensteiner M*, Misgeld T. Near-infrared branding efficiently correlates light and electron microscopy. Nat Methods. 2011 Jun 5;8(7):568-70.. (* co-senior author)
Gilley J, Seereeram A, Ando K, Mosely S, Andrews S, Kerschensteiner M, Misgeld T, Brion JP, Anderton B, Hanger DP, Coleman MP. Age-dependent axonal transport and locomotor changes and tau hypophosphorylation in a "P301L" tau knockin mouse. Neurobiol Aging. 2012 Mar;33(3):621.
Bareyre FM, Garzorz N, Lang C, Misgeld T, Büning H, Kerschensteiner M. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc Natl Acad Sci U S A. 2011 Apr 12;108(15):6282-7.
Nikić I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, Brück W, Bishop D, Misgeld T, Kerschensteiner M. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2011 Apr;17(4):495-9.


Martin Kerschensteiner, Principal Investigator
Read more about the PI on the next tab.

Daniela Beckmann, PhD student
I studied Molecular Medicine at Ulm University in a Bachelor- and consecutive Master programme. During this time, I became particularly interested in both neuroscience and immunology, which is why I happily ended up as a PhD student in Neuroimmunology and joined the Kerschensteiner Lab in October 2019. I study the calcium-mediated process of axon degeneration in inflammatory lesions, using transgenic and in vivo imaging techniques. To pursue this PhD project, I was awarded a scholarship by the “Studienstiftung des deutschen Volkes” (German Academic Scholarship Foundation). Whenever I am not in the lab, you will find me playing clarinet in a symphonic wind orchestra or reading a good book.

Clara de la Rosa del Val, PhD student
I studied Biochemistry at the Autonomous University of Madrid, in Spain, doing a one-year exchange at the University of Toronto, Canada. I really liked the international experience, so for my master I decided to move abroad to Munich. I studied a MSc of Neuroscience with the Graduate School of Systemic Neurosciences (GSN) at the Ludwig-Maximilians Universität. During my master studies, I already joined the Kerschensteiner Lab, first for a research internship and later for the Master Thesis. Now I’m continuing with the PhD, also as part of the GSN. I work on the functional regulation of phagocytes and T cells in neuroinflammation, using the CRISPR/Cas9 technology in CRISPR screens. In my free time, I like to read, paint, hike and go to the Alps (hands down one of the best things about Munich), read some more and have fun with friends.

Bernadette Fiedler, Technical Assistant
I have been in Martin`s lab for more than 10 years by now and as a technician I am responsible for genotyping, ordering and managing the lab. As the mum of 2 soccer crazy boys I am trying to coordinate their game schedules with the experiments in the lab.

Dr Arek Kendirli, postdoctoral fellow
I studied Biology at Middle East Technical University (METU). During my bachelor's, I worked in Kocer's lab at the University of Groningen and Lemaitre's lab at École polytechnique fédérale de Lausanne (EPFL). Then I moved to Germany for my master's degree in cancer biology at the German Cancer Research Center (DKFZ) the University of Heidelberg. During my master's, I worked in Lander's lab at Broad Institute of MIT and Harvard and in Boutros' lab at DKFZ. For my Ph.D., I joined the Kerschensteiner's lab to apply loss-of-function CRISPR screens in immune cells in the concept of multiple sclerosis. I would say the most efficient sgRNA is the one that hasn't been designed yet. Outside the lab, playing football and playing cajón are my favorite activities.

Emily Melisa Ullrich Gavilanes, Post-doctoral fellow
Born and raised in Quito, Ecuador, I moved to Germany for my bachelor’s degree in Biochemistry and Cell Biology at Jacobs University Bremen. I then obtained a Master in Cellular and Molecular Neuroscience at Karl Eberhardt Universität Tübingen before moving to Munich to work on my PhD in the Kerschensteiner lab in November 2018. My project focuses on the mechanisms that lead to grey matter spine and neuron loss in a model of cortical multiple sclerosis. Outside of the lab I enjoy spending my time doing sports like skiing, tennis, swimming or I enjoy nice days with walks around the city parks and lakes.

Dr. Yi-Heng Tai, Postdoctoral fellow
After my master’s training in brain science in Taiwan, I did my PhD here focusing on the mitochondrial pathology in an immune-mediated neurodegenerative model. It is quite fascinating to make great effort on staring at this tiny subcellular structure- imagine how important this organelle is and how they affect your life (#powerhouseisonfire hashtag years spent for my PhD). Coming from subtropics, I fell deeply in love with the winter here in Munich. The alps also made me an ‘’outdoor person’’. No doubt that I hike and enjoy the beer on top of the mountain. Apart from outdoor activities, I travel, cook and bake (like a lot). And just fyi- if you would like to bring some sweets for me, I am a Krapfen lover!

Nils Treiber, MD student
I have been studying medicine at the LMU/TUM since 2018 and got to the Kerschensteiner lab initially through the `LMU-Forschungsmodul´ as an intern. Since then I have been introduced to great many techniques and surgeries while helping to research progressive MS models. After my preclinical phase I will be doing my MD on my current subject of progressive forms of MS. In my medical studies I was always fascinated by neurological disease, which is why I am excited to contribute to better understanding them. I originally come from a rather small town near the alps, which is why I really enjoy going hiking and skiing, knowing the secret tips. In the rest of my free time I also like working and troubleshooting (Pfusch) on my old car, playing dungeons and dragons with friends and riding my (slightly too fast) bike.

Ionna Emmanouilidis, PhD student
I studied Biology at Pierre and Marie Curie University in Paris and spend a semester abroad at Karolinska Institute in Stockholm. I moved to Munich to study at the Graduate School of Systemic Neurosciences and obtained an MSc of Neuroscience. I joined the Kerschensteiner lab in October 2019 for a short internship, which turned into a master thesis, which then turned into a PhD project that I do in both the Kerschensteiner and the Misgeld lab (Technical University of Munich). My PhD project´s aim is to reveal the mechanisms underlying plasma membrane repair in porated axons in the context of neuroinflammation and spinal cord contusion injury. To address this question I use methods such as in vivo 2-photon imaging and CRISPR manipulations. Born and raised in Greece I prefer the sea over the Bavarian Alps and summer over winter. I spend a lot of my free time reading or discovering the city with my friends, so ask me if you ever need `where to go in Munich´ advice.

Veronika Pfaffenstaller, PhD student
I studied Biology and the consecutive Master Human Biology - Principles of Health and Disease at the Ludwig-Maximilians Universität München. I joined the Kerschensteiner lab for a research internship and later for my Master Thesis. I will continue with my PhD working on identifying new therapeutic target structures for the modulation of phagocyte function in neuroinflammation and to validate them functionally, molecularly and pharmacologically using the CRISPR/Cas9 technology. Outside the lab, I enjoy sports like running, swimming and ballet, or I like reading and playing dungeons and dragons with friends (yes we also have a lab dnd group).

Yves Carpentier Solorio, PhD student
Born in Mexico and raised in Montreal, Canada, I did a bachelor and a master’s degree in Neurosciences at the University of Montreal. I joined the Kerschensteiner lab in September 2022 to do my PhD to pursue my interest in neuroimmunology. My project focuses on the mechanisms of axonal degeneration in the spinal cord in a mouse model of MS using CRISPR/Cas9 technology. During my free time, you can find me on a tennis court, cooking or simply on my couch with a charcuterie board and a rosé.

Victoria Böck, Institute Technical Assistant
I was born in Munich and i still love to live in the capital of bavaria. After secondary school i did an apprentice ship to become a MTLA (medical-technical-laboratory-assistant), which i completed with a state examination. I worked in a routine microbiology lab for a few years before moving into research. My first research experience was in biochemistry and molecular biology, after which I switched to neuroimmunology, into the Institute of clinical neuroimmunology, where I really enjoy working. In my free time, I try to be outside as much as possible, for example riding my motorcycle, travel around the world or hiking in the mountains. At home I sew myself useful things or read technical literature.

Carla Ares Carral, PhD student
Made in Spain. After getting my bachelors degree in Biochemistry at the Universidad Complutense de Madrid, I moved to München because I heard beers and the Alps were nice. Now I can say… combined are better. After doing a research internship at Kerschensteiner lab as part of the LMU Human Biology - Principles of Health and Disease Master Program, I decided I enjoyed it too much not to stay. First it was just for the master thesis. Now for a PhD, which focuses on the identification of molecular signals that mediate adaptive instruction of innate effector function in MS. When I’m not bothering my coworkers at the BMC, you’ll probably find me running around the city, grabbing coffee with friends or making tortilla de patata.

Anastasiia Sydorenko, MD student
Born and raised in Ukraine, Kiew, I moved to Munich to study biochemistry (TUM). During my bachelor’s a wish to combine both fascination in science and direct patient’s care led to my decision to study medicine (LMU) while continuing with my master’s in biochemistry (TUM). Having developed a strong interest in both neurobiology and immunology I joined the Kerschensteiner Lab first for a research internship during my master’s and I am now happy to be back for my medical thesis. To pursue my MD I was awarded a scholarship by the Hertie foundation. In my project I study the role of pyroptosis players gasdermin D and E in the axon degeneration in inflammatory lesions of a mouse model of multiple sclerosis using transgenic and in vivo imaging. Whenever I'm not in the lab, I enjoy sports like hiking, climbing and skiing (well, still learning it, but being passionate), dancing (ballet and modern), acting in an amateur theater or just reading a good book.

Prof. Dr. med. Martin Kerschensteiner
I started to get interested in neuroimmunology early on in my medical studies. Therefore I moved from Aachen to Munich to join Hartmut Wekerle`s department at the Max Planck Institute for Neurobiology, where I worked for 6 years in parallel to my medical studies. At the time it became clear that axonal injury was a critical determinant of disability in MS patients and I wanted to learn more about neuroscientific approaches to axonal pathology. Supported by a postdoctoral fellowship I therefore joined the lab of Martin Schwab at ETH Zurich and developed first targeted EAE models that allowed the induction of neuroinflammatory lesions in a defined axonal tract system (and subsequent analysis of axonal injury and compensation).
During this time I also visited a friend of mine (Thomas Misgeld), then a post doc in Jeff Lichtman`s lab at Washington university in St. Louis, and we managed to obtain first in vivo images of fluorescently labeled axons in the spinal cord. Based on this we were able to secure funding that allowed me to join Jeff`s lab first at Wash U and then at Harvard and enabled us to establish imaging strategies to study axonal pathology in real time. Support from the Emmy Noether program of the DFG then made it possible for me to start my own research group at the Institute of Clinical Neuroimmunology of the LMU Munich. Since then I have tried to apply dynamic imaging approaches to better understand the cellular and molecular interactions that determine the fate of neuroinflammatory lesion, first as a group leader, then as an associate professor and since 2013 as full professor.
As the director of the Institute of Clinical Neuroimmunology I hope to be able to continue the groundbreaking work of my mentors Reinhard Hohlfeld and Hartmut Wekerle and aim to provide an environment that stimulates scientific independence and creativity and hopefully contributes to meaningful improvements of our understanding of neuroinflammatory conditions and our means to diagnose, monitor and treat them.
Person
Name: Prof. Dr. med. Martin Kerschensteiner
Education and Academic Appointments
Since 2013 Full professor and Director of the Institute of Clinical Neuroimmunology, Medical Center of the LMU Munich
2008 – 2013 Associate Professor for Translational Neuroimmunology at the Institute of Clinical
2005 – 2008 Group leader funded by the Emmy Noether-Program of the German Research Foundation (DFG), Institute of Clinical Neuroimmunology, Medical Center oft he Ludwig-Maximilians-University Munich (LMU Munich)
2004 – 2005 Research Associate in the Department of Molecular and Cellular Biology (lab of Prof. J. Lichtman), Harvard University, Cambridge, USA
2003 – 2004 Research Associate in the Department of Anatomy and Neurobiology (lab of Prof. J. Lichtman), Washington University School of Medicine, St. Louis, USA
2001 – 2003 Post-doctoral fellow in the Department of Neuromorphology (director: Prof. M. Schwab), Brain Research Institute, University and ETH Zurich, Switzerland
1999 – 2001 Residency training in Neurology, University Hospital LMU Munich
2000 Medical degree (Dr. med.; summa cum laude)
1994 – 1999 MD thesis at the Department of Neuroimmunology (director: Prof. H. Wekerle), Max-Planck-Institute of Neurobiology, Martinsried
1992 – 1999 Medical Studies at the RWTH Aachen and the LMU Munich
Coordinating Functions
Since 2020 Deputy Speaker of the Collaborative Research Center SFB-TRR 274 “Checkpoints of CNS recovery”
Since 2020 Head of the Examination Board, Elite Master Program “Human Biology –Principles of Health and Disease”, LMU Munich
Since 2017 Deputy Chair of the Biomedical Center of the LMU Munich
Since 2016 Board Member of the Clinical Compentence Network “Multiple Sclerosis”
Since 2016 Board Member of the Collaborative Research Center SFB-TRR 128 “Multiple Sclerosis –Initaiting/effector vs. regulatory mechanisms”
Since 2012 Board Member and Research Focus Coordinator of the Excellence Cluster “Munich Cluster for Systems Neurology (SyNergy)”
Awards and Honors
2023 Research awaes for multiple sclerosis research of the Sobek foundation
2020 Elected member of the German National Academy of Sciences (Leopoldina)
2012 ERC Consolidator Grant awarded by the European Research Council
2008 “Habilitationsförderpreis“ of the LMU Munich
2005 Independent group leader award of the DFG Emmy-Noether Program
2004 1st Wyeth Award for MS Research presented by the German Neurological Society
1994 1st Junior Research Award of the German Multiple Sclerosis Society (“Sobek Price“)
1994 – 1999 Scholar of the German National Scholarship Foundation (“Studienstiftung des deutschen Volkes e.V.“)

2023 Christmas party

2023 Lab retreat

2023 Christmas party

2023 Lab retreat

Lab dinner 2023

2023 Christmas party

2023 Lab retreat


2022 Arek's defense

2022 Daniel's defense

2022 Lab retreat

2022 Oktoberfest

2022 Arek's wedding

2022 Dinner night

Mehrnoosh farewel

Lab retreat 2022

Bayern Barcelone Women Football

2022 Lab retreat- after party


2020 Yi-Heng PhD defense

2020 Kerschensteiner Group Picture

2019 Sunset at BMC

2018 Marta PhD defense

2017 Dumplings at the BMC

2017 Christmas party

2016 Christmas party

2020 Zala Farewell party

2019 Hiking trip

2018 Escape room

2017 Delphine PhD defense

2016 Elisa Phd defense

2020 Tapas night

2019 Oktoberfest

2018 Lab BBQ at Berni`s

2017 Delphine PhD defense

2016 Oktoberfest
We gratefully acknowledge support for our work by the following agencies:

FOR5705 Neuroflame – German Research Foundation (DFG)
More

Munich Cluster for Systems Neurology (SyNergy)

Collaborative Research Center SFB-TR 128

LMU Sanofi Research Collaboration

Deutsche Multiple Sklerose Gesellschaft Bundesverband e.V.

Gemeinnützige Hertie-stiftung

Studienstiftung des deutschen Volkes e.V.

Verein zur Therapieforschung für MS Kranke




