Publications

Multiple sclerosis and gut microbiota: Lachnospiraceae from the ileum of MS twins trigger MS-like disease in germfree transgenic mice
Press release: https://www.lmu.de/de/newsroom/newsuebersicht/news/multiple-sklerose-ausloeser-in-der-darmflora.html
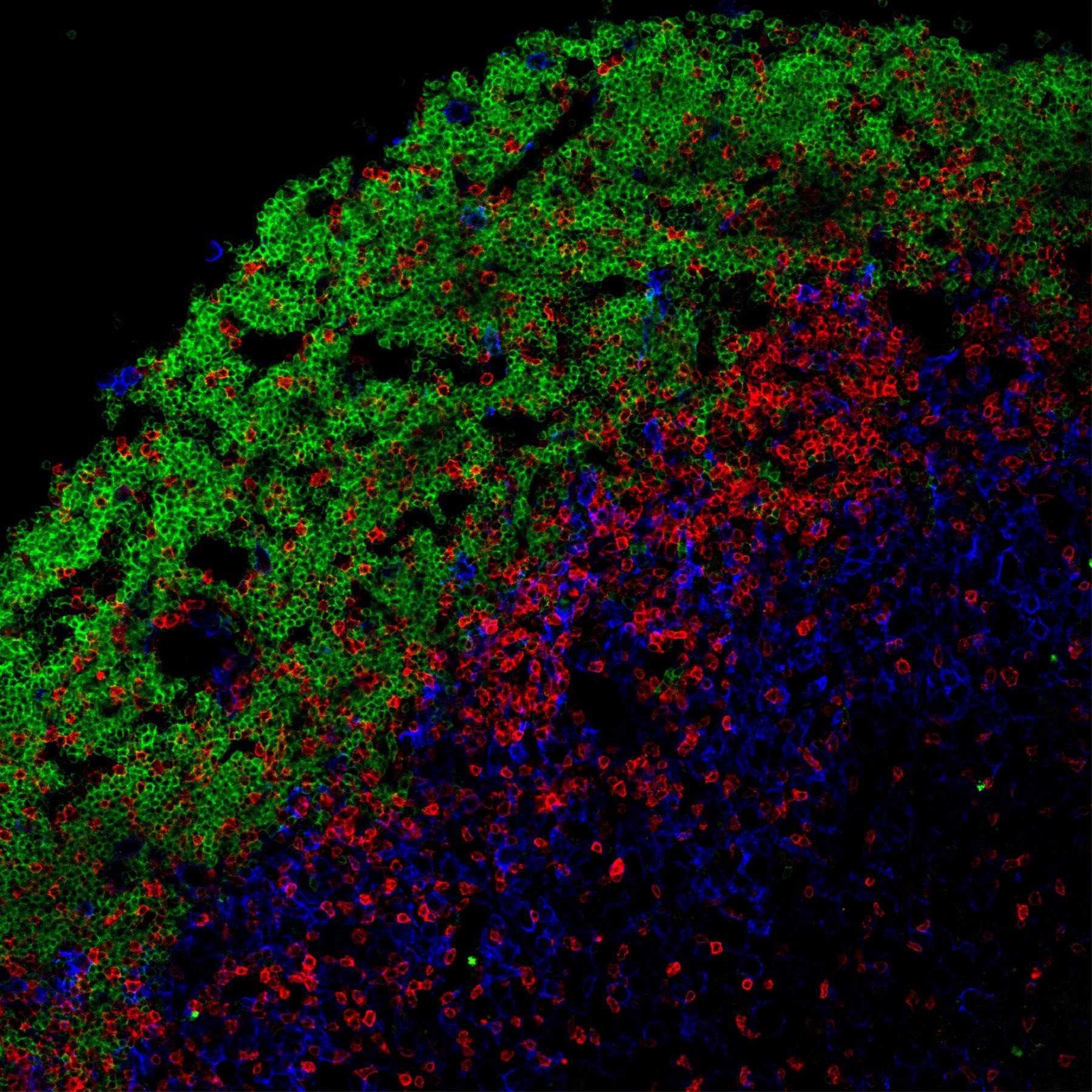
T–B cell cooperation in ectopic lymphoid follicles propagates CNS autoimmunity
Press release: https://www.lmu.de/en/newsroom/news-overview/news/how-b-and-t-cells-fuel-the-pathological-process-in-ms.html

LMU researchers demonstrate that certain immune cells already play an important role in the early stages of multiple sclerosis

Fluorogenic chemical probes for wash-free imaging of cell membrane damage in ferroptosis, necrosis and axon injury

A B cell–driven EAE mouse model reveals the impact of B cell–derived cytokines on CNS autoimmunity

A genome-wide in vivo CRISPR screen identifies essential regulators of T cell migration to the CNS in a multiple sclerosis model.
Kendirli A, de la Rosa C, Lämmle KF, Eglseer K, Bauer IJ, Kavaka V, Winklmeier S, Zhuo L, Wichmann C, Gerdes LA, Kümpfel T, Dornmair K, Beltrán E, Kerschensteiner M, Kawakami N. (2023) A genome-wide in vivo CRISPR screen identifies essential regulators of T cell migration to the CNS in a multiple sclerosis model. Nat Neurosci. 26(10):1713-1725.
Press release : https://www.lmu.de/en/newsroom/news-overview/news/identified-key-regulators-involved-in-genesis-of-multiple-sclerosis-lesions.html

Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions.
Tai YH, Engels D, Locatelli G, Emmanouilidis I, Fecher C, Theodorou D, Müller SA, Licht-Mayer S, Kreutzfeldt M, Wagner I, de Mello NP, Gkotzamani SN, Trovò L, Kendirli A, Aljović A, Breckwoldt MO, Naumann R, Bareyre FM, Perocchi F, Mahad D, Merkler D, Lichtenthaler SF, Kerschensteiner M, Misgeld T. (2023). Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions. Nature metabolism, 5(8), 1364–1381.
Press release Synergy: https://www.synergy-munich.de/news/news/kerschensteiner-misgeld/index.html

Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex
Mezydlo A, Treiber N, Ullrich Gavilanes EM, Eichenseer K, Ancău M, Wens A, Ares Carral C, Schifferer M, Snaidero N, Misgeld T, Kerschensteiner M. Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron. 2023 Apr 13:S0896-6273(23)00227-1.

New insights into effector functions of autoantibodies to myelin oligodendrocyte glycoprotein
Mader S, Ho S§, Wong H K§, Baier S§, Winklmeier S, Riemer C, Rübsamen H, Fernandez I, Gerhards R , Du C, Chuquisana Omar, Lünemann J, Lux A, Nimmerjahn F, Bradl M*, Kawakami N*, Meinl E* Dissection of complement and Fc-receptor-mediated pathomechanisms of autoantibodies to myelin oligodendrocyte glycoprotein, Proc Natl Acad Sci U S A. 2023 Mar 28; 120(13):e2300648120.
Press release - LMU: https://www.lmu.de/de/newsroom/newsuebersicht/news/autoimmunerkrankung-mogad-neue-einblicke-in-die-pathomechanismen.html

Anti-CD20 therapy in multiple sclerosis: effects beyond B cell depletion
January 2023- Therapies with anti-CD20 mAbs are beneficial in multiple sclerosis patients, commonly attributed to depletion of B cells. We (the Mader and Meinl labs together with the clinical Kümpfel team and collaborators) found a further effect of anti-CD20 on the immune system, namely reduction of the free soluble receptor and decoy sTACI due to complex formation with its ligand BAFF. Since a previous clinical trial revealed that a pharmacological variant of sTACI (TACI-Fc; atacicept) unexpectedly worsened MS, the reduction of the endogenous sTACI might contribute to the beneficial effect anti-CD20 in MS by enhancing the activity of APRIL (proposed mechanism illustrated in our cartoon).
Figure Legend: The upper part shows our findings: ocrelizumab (anti-CD20) treatment results in an increase of BAFF, formation of sTACI-BAFF complexes and reduction of free sTACI. The lower part links our findings to published work: sTACI is a decoy for APRIL (left). In the absence of the decoy sTACI (right), APRIL might induce IL10 production from astrocytes and promote the development of regulatory IgA plasma cells, which can home to the brain and secrete IL-10.
Ho S, Oswald E, Wong HK, Vural A, Yilmaz V, Tüzün E, Türkoğlu R, Straub T, Meinl I, Thaler F, Kümpfel T, Meinl E*, Mader S*. Ocrelizumab Treatment Modulates B-Cell Regulating Factors in Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm. 2023 Jan 26;10(2):e200083.

Synaptogenic gene therapy with FGF22 improves circuit plasticity and functional recovery following spinal cord injury
January 2023- The Bareyre Lab establishes FGF22 gene therapy as a new synaptogenic treatment that improves neuronal rewiring and functional recovery following spinal cord injury and determines the temporal constraints for its application in a paper published in EMBO Molecular Medicine.
• Following incomplete spinal cord injury, transected motor circuits rewire to contribute to functional recovery.
• FGF22 gene therapy induces synaptogenesis and allows targeted support of neuronal rewiring following spinal cord injury.
• Delivery of FGF22 gene therapy acutely and up to 1 day following spinal cord injury improves functional recovery while delayed application fails to do so.
Aljović A, Jacobi A, Marcantoni M, Kagerer F, Loy K, Kendirli A, Bräutigam J, Fabbio L, Van Steenbergen V, Pleśniar K, Kerschensteiner M, Bareyre FM. Synaptogenic gene therapy with FGF22 improves circuit plasticity and functional recovery following spinal cord injury. EMBO Mol Med. 2023 Jan 5:e16111.

Coordinated neurostimulation promotes circuit rewiring and unlocks recovery after spinal cord injury
December 2022- Functional recovery after incomplete spinal cord injury depends on the effective rewiring of neuronal circuits. Here the Bareyre Lab shows in a paper published by the Journal of Experimental Medicine that selective chemogenetic activation of either corticospinal projection neurons or intraspinal relay neurons alone led to anatomically restricted plasticity and little functional recovery. In contrast, coordinated stimulation of both supraspinal centers and spinal relay stations resulted in marked and circuit specific enhancement of neuronal rewiring, shortened EMG latencies and improved locomotor recovery. Taken together, our study demonstrates that a refined anatomical understanding of the neuronal circuits that reform in the injured CNS, empowers the design of targeted multi-level neurostimulation strategies that selectively foster adaptive circuit rewiring and thereby unlock the CNS´s potential for recovery.
Van Steenbergen V, Burattini L, Trumpp M, Fourneau J, Aljović A, Chahin M, Oh H, D'Ambra M, Bareyre FM. Coordinated neurostimulation promotes circuit rewiring and unlocks recovery after spinal cord injury. J Exp Med. 2023 Mar 6;220(3):e20220615.

Broader Epstein–Barr virus–specific T cell receptor repertoire in the MS TWIN STUDY
September 2022- Epstein–Barr virus (EBV) infection precedes multiple sclerosis (MS) pathology and cross-reactive antibodies might link EBV infection to CNS autoimmunity. As an altered anti-EBV T cell reaction was suggested in MS, in collaboration with the Institute of Translational Neurology in Münster, we queried peripheral blood T cell receptor β chain (TCRβ) repertoires in 35 monozygotic, MS-discordant twin pairs and larger control groups for multimer-confirmed, viral antigen–specific TCRβ sequences. We detected more MHC-I–restricted EBV-specific TCRβ sequences in MS patients. In MS patients, cerebrospinal fluid also contained EBV-specific central memory CD8+ T cells, suggesting recent priming. Therefore, MS is not only preceded by EBV infection, but also associated with broader EBV-specific TCR repertoires, consistent with an ongoing anti-EBV immune reaction in MS.
Schneider-Hohendorf T*, Gerdes LA*, Pignolet B, Gittelman R, Ostkamp P, Rubelt F, Raposo C, Tackenberg B, Riepenhausen M, Janoschka C, Wünsch C, Bucciarelli F, Flierl-Hecht A, Beltrán E, Kümpfel T, Anslinger K, Gross CC, Chapman H, Kaplan I, Brassat D, Wekerle H, Kerschensteiner M, Klotz L, Lünemann JD, Hohlfeld R, Liblau R, Wiendl H, Schwab N. Broader Epstein-Barr virus-specific T cell receptor repertoire in patients with multiple sclerosis.J Exp Med. 2022;219(11):e20220650.

Longitudinal retinal changes in MOGAD
July 2022 – In contrast to multiple sclerosis, it was not sufficiently investigated whether patients with myelin-oligodendrocyte-glycoprotein-antibody (MOG-IgG)-associated disease (MOGAD) also show a relapse-independent, subclinical retinal neuro-axonal loss. Therefore, initiated by @NeuroVisionLab (Havla Lab), eighty MOGAD patients and 139 healthy controls (HC) were included in a worldwide multicenter study with longitudinal optical coherence tomography (OCT) data. Longitudinally (follow-up up to 3 years), our analysis showed no attack-independent retinal damage in MOGAD. Nevertheless, ongoing neuroaxonal damage or edema resolution appears to occur up to 12 months after optic neuritis (ON), which is longer than has been reported for other forms of ON. These findings suggest that the pathomechanisms underlying optic nerve involvement and the development of OCT retinal changes after ON are different in MOGAD.
Oertel FC*, Sotirchos ES*, Zimmermann HG, Motamedi S, Specovius S, Asseyer ES, Chien C, Cook L, Vasileiou E, Filippatou A, Calabresi PA, Saidha S, Pandit L, D'Cunha A, Outteryck O, Zéphir H, Pittock S, Flanagan EP, Bhatti MT, Rommer PS, Bsteh G, Zrzavy T, Kuempfel T, Aktas O, Ringelstein M, Albrecht P, Ayzenberg I, Pakeerathan T, Knier B, Aly L, Asgari N, Soelberg K, Marignier R, Tilikete CF, Calvo AC, Villoslada P, Sanchez-Dalmau B, Martinez-Lapiscina EH, Llufriu S, Green AJ, Yeaman MR, Smith TJ, Brandt AU, Chen J*, Paul F*, Havla J*; with the GJCF International Clinical Consortium for NMOSD and the CROCTINO study group. Longitudinal retinal changes in MOGAD. Ann Neurol. 2022 Jun 15. doi: 10.1002/ana.26440. Epub ahead of print.

Selective plasticity of callosal neurons in the adult contralesional cortex following murine traumatic brain injury
May 2022 – The Bareyre lab studied which contralesional circuits adapt following traumatic brain injury (TBI). Laura Empl and Alexandra Chovsepian used in vivo imaging, retrograde labeling, rabies tracing, tissue clearing and functional imaging to demonstrate in Nature Communications that callosal neurons selectively adapt after TBI in mice..
Traumatic brain injury (TBI) results in deficits that are often followed by recovery. The contralesional cortex can contribute to this process but how distinct contralesional neurons and circuits respond to injury remains to be determined. To unravel adaptations in the contralesional cortex, we used chronic in vivo two-photon imaging. We observed a general decrease in spine density with concomitant changes in spine dynamics over time. With retrograde co-labeling techniques, we showed that callosal neurons are uniquely affected by and responsive to TBI. To elucidate circuit connectivity, we used monosynaptic rabies tracing, clearing techniques and histology. We demonstrate that contralesional callosal neurons adapt their input circuitry by strengthening ipsilateral connections from pre-connected areas. Finally, functional in vivo two-photon imaging demonstrates that the restoration of pre-synaptic circuitry parallels the restoration of callosal activity patterns. Taken together our study thus delineates how callosal neurons structurally and functionally adapt following a contralateral murine TBI.
Empl, L., Chovsepian, A., Chahin, M. et al. Selective plasticity of callosal neurons in the adult contralesional cortex following murine traumatic brain injury. Nat Commun 13, 2659 (2022).

MS discordant monozygotic twins help to untangle environmental and genetic influences in immune profiles
February 2022 – The Beltrán and Gerdes labs studied the immune profiles in the MS TWIN STUDY to differentiate the influence of the environment and genetics in multiple sclerosis. In collaboration with the Becher lab from the University of Zurich they may have discovered precursor cells of the disease-causing T cells.
The impact of genetic risk has been studied for years, however the genetic background is far from deterministic and interaction with environmental factors is needed for MS to evolve. However, which part of the aberrant immune response is guided by either genes or environment is difficult to decipher. With the help of the unique setting of the MS TWIN STUDY we could show that about half of the composition of our immune system is determined by the genetic background. In this project, peripheral blood mononuclear cells of 61 pairs of identical twins with discordance for MS were examined with the use of cutting-edge single-cell technologies and artificial intelligence to describe the immune profiles of the twin pairs in rich detail and with full control of genetic influences. Thereby we could not only identify characteristic proteins in the immune cells of the MS affected twin, but also decode which transcriptional programs are switched on in these cells. Specifically, in the blood of MS twins we detected T cells with characteristics of recently activated cells, which are more likely to migrate into the central nervous system and cause damage there.
Ingelfinger F*, Gerdes LA*, Kavaka V, Krishnarajah S, Friebel E, Galli E, Zwicky P, Furrer R, Peukert C, Dutertre CA, Eglseer KM, Ginhoux F, Flierl-Hecht A, Kümpfel T, De Feo D, Schreiner B, Mundt S, Kerschensteiner M, Hohlfeld R, Beltrán E#, Becher B#. Twin study reveals non-heritable immune perturbations in multiple sclerosis. Nature. 16 February 2022. DOI: 10.1038/s41586-022-04419-4

PET imaging can improve montoring of Natalizumab-associated PML
November 2021 – Progressive multifocal leukoencephalopathy (PML) is a severe CNS infection that can occur in MS patients treated with Natalizumab. Clinical management of patients with Natalizumab-associated PML is challenging not least to detect, monitor and differentiate PML lesions are limited. Here the Kerschensteiner lab and Kümpfel group teamed up to investigate whether TSPO PET imaging can be applied to monitor the inflammatory activity of PML lesions over time. The results of this monocentre pilot study now indicate that TSPO PET imaging may faciliate longitudinal monitoring of disease activity and help distinguish recurrent multiple sclerosis activity from PML progression.
Mahler C, Schumacher AM, Unterrainer M, Kaiser L, Höllbacher T, Lindner S, Havla J, Ertl-Wagner B, Patzig M, Seelos K, Neitzel J, Mäurer M, Krumbholz M, Metz I, Brück W, Stadelmann C, Merkler D, Gass A, Milenkovic V, Bartenstein P, Albert NL, Kümpfel T, Kerschensteiner M. TSPO PET imaging of natalizumab-associated progressive multifocal leukoencephalopathy. Brain. 2021 Oct 22;144(9):2683-2695.

Secret, highly active life of nerve cell contacts in the brain revealed
June 2021 – Nerve cells in the brain possess small protrusions, so called dendritic spines, to communicate with other nerve cells by forming a synapse. These contact sites can be both, short- or long-lived thereby existing for even up to an entire lifetime. Short-lived contact sites reflect an ever- adapting brain, which is necessary to process novel experiences. Long-lived, stable contact sites, on the other hand, form the basis of our memory. But even stable memory content can vary to some extent over time. The basis of this process still remains incompletely understood. The work, published in the journal Science Advances, shows that although a subset of synaptic structures remain present at the same location over a period of at least one month, their subunits underwent a remarkable and unexpected strong change in size and shape. Synaptic structures are thus both stable and dynamic – 'volatile' – at the same time, offering a glimpse into the complexity of synaptic remodelling and computational power mammalian brains are endowed with. Press release
Steffens H, Mott AC, Li S, Wegner W, Svehla P, Kan WYV, Wolf F, Liebscher S# & Willig KI# (2021). Stable but not rigid: Chronic in vivo STED nanoscopy reveals extensive remodeling of spines, indicating multiple drivers of plasticity. Science Adv Jun 9;7(24) # equal contr

Details of target-recognition by autoantibodies to myelin oligodendrocyte glycoprotein uncovered
June 2021 – Antibodies (Abs) to myelin oligodendrocyte glycoprotein (MOG) define a distinct disease entity, MOGAD. We report that the intracellular part of MOG, specifically its second hydrophobic domain enhances the recognition of the extracellular part of MOG by autoantibodies from patients. This explains the need for a cell-based assay to identify patients with MOG-Abs. We further found that MOG-Abs from patients require bivalent binding to recognize MOG. Since bivalently bound MOG-Abs poorly bind C1q, this indicates that complement-mediated demyelination is not the major pathomechanism of MOG-Abs.
Macrini C, Gerhards R, Winklmeier S, Bergmann L, Mader S, Spadaro M, Vural A, Smolle M, Hohlfeld R, Kümpfel T, Lichtenthaler S, Franquelim H, Jenne D, Meinl E. Features of MOG required for recognition by patients with MOG-antibody-associated disorders. Brain, epub, 2021.
Video: Features of MOG required for recognition by patients with MOG antibody-associated disorders

Synaptic FUS(S) at the heart of neuronal dysfunction in ALS/FTD
June 2021 – The Liebscher lab together with the Dupuis group in Strasbourg, France and a highly collaborative international team of several other groups have found the missing pieces between genetic mutations linked to the development of Amyotrophic lateral sclerosis and Frontotemporal Dementia and the consecutive behavioral deficits. Using a wide array of methodological approaches they found that cytoplasmic mislocalization of the protein Fused-in-Sarcoma (FUS) causes its synaptic accumulation and local transcriptional dysregulation, leading to synaptic deficits of primarily inhibitory synapses in frontal cortex, which is causing neuronal hyperexcitability and consequently behavioral alterations. These data thus indicate that cytoplasmic FUS can excert deleterious effects beyond motor neuron degeneration, which are mainly caused by synaptic alterations.
Jelena Scekic-Zahirovic*, Inmaculada Sanjuan-Ruiz*, Vanessa Kan, Salim Megat, Pierre De Rossi, Stéphane Dieterlé, Raphaelle Cassel, Marguerite Jamet, Pascal Kessler, Diana Wiesner, Laura Tzeplaeff, Valérie Demais, Sonu Sahadevan, Katharina M. Hembach, Hans-Peter Muller, Gina Picchiarelli, Nibha Mishra, Stefano Antonucci, Sylvie Dirrig-Grosch, Jan Kassubek, Volker Rasche, Albert Ludolph, Anne-Laurence Boutillier, Francesco Roselli, Magdalini Polymenidou, Clotilde Lagier-Tourenne, Sabine Liebscher# and Luc Dupuis#, Cytoplasmic FUS triggers early behavioral alterations linked to cortical neuronal hyperactivity and inhibitory synaptic defects, Nature Communications, 2021,12:3028

An encephalitogenic autoantibody cross-reacts to a tumor antigen
March 2021 – The Dornmair group together with the Meuth/Melzer group from Münster, Germany and several other colleagues investigated antibodies recognizing the neuronal gamma-aminobutyric-acid-A receptor (GABAA-R), which cause severe encephalitis. From the cerebrospinal fluid of a patient with GABAA–R encephalitis, they cloned a highly expressed antibody and showed that it binds the GABAA-R and influences signal transduction in neurons explaining clinical symptoms. They confirmed that the antibody cross-reacts to an onco-protein, which is known to be involved in several malignancies and showed that cross-reactivity to this onco-protein may also be detected in two other GABAA-R patients. This suggests that such cross-reactivity is presumably a key event in the pathogenesis of GABAA-R encephalitis.
Brändle SM, Cerina M, Weber S, Held K, Menke AF, Alcalá C, Gebert D, Herrmann AM, Pellkofer H, Gerdes LA, Bittner S, Leypoldt F, Teegen B, Komorowski L, Kümpfel T, Hohlfeld R, Meuth SG, Casanova B, Melzer N, Beltrán E, Dornmair K. Cross-reactivity of a pathogenic autoantibody to a tumor antigen in GABAA receptor encephalitis. Proc Natl Acad Sci U S A. 2021 Mar 2;118(9):e1916337118.

Immune cells remove synapses in the inflamed gray matter
January 2021 – Gray matter pathology is a critical contributor to disability in advanced stages of multiple sclerosis. Here the Kerschensteiner lab teamed up with the Misgeld and Merkler lab to investigate how neuronal structure and function is impacted in the neuronal gray matter. In a mouse model they show that gray matter inflammation leads to a synapse loss that is accompagnied by neuronal silencing and can be reversible. Synapse loss is primed by local calcium increases, executed by activated microglial cells and infiltrating monocyte-derived macrophages and can be mitigated by therapeutic strategies that interfer with pathological activation of phagocytes. The authors hope that their work can help the development of therapeutic approaches that curb progression in MS patients.
Jafari M, Schumacher AM, Snaidero N, Ullrich Gavilanes EM, Neziraj T, Kocsis-Jutka V, Engels D, Jürgens T, Wagner I, Weidinger JDF, Schmidt SS, Beltrán E, Hagan N, Woodworth L, Ofengeim D, Gans J, Wolf F, Kreutzfeldt M, Portugues R, Merkler D, Misgeld T, Kerschensteiner M. Phagocyte-mediated synapse removal in cortical neuroinflammation is promoted by local calcium accumulation. Nat Neurosci. 2021 Mar;24(3):355-367

Resurrection of a pathogenic immune response from a historical case
January 2021 – The Dornmair group together with the Lassmann/Bradl group from the Medical University of Vienna and colleagues of the Meinl and Kawakami groups analyzed a historic case of a patient who died 60 years ago from a disease closely resembling multiple sclerosis, following a misguided immunization with lyophilized calf brain tissue. Transcriptome analyses by mRNAseq and antibody reconstruction by bioinformatics revealed an autoantibody (rAb-hAE) against myelin oligodendrocytes glycoprotein (MOG), similar to the encephalitogenic anti-MOG antibody 8-18C5. Both antibodies induced demyelination after injection into animals, whereas the negative control antibody rMS3-s1 did not. This “archeological neuroimmunology” approach shows that autoimmunization with brain tissue in humans may induce a disease that is similar to multiple sclerosis.
Beltrán E, Paunovic M, Gebert D, Cesur E, Jeitler M, Höftberger R, Malotka J, Mader S, Kawakami N, Meinl E, Bradl M, Dornmair K, Lassmann H. Archeological neuroimmunology: resurrection of a pathogenic immune response from a historical case sheds light on human autoimmune encephalomyelitis and multiple sclerosis. Acta Neuropathol. 2021 Jan;141(1):67-83.

Single cell ablation reveals the rules of myelin replacement
September 2020 – To interrogate the rules that govern myelin replacement in the cortex the Kerschensteiner group together with the Misgeld lab at TUM studied the response to the ablation of single cortical oligodendrocytes. Using timelapse imaging and correlated ultrastructural reconstructions they were able to show that a loss of a single oligodendrocyte is sufficient to cause robust cell and myelin replacement. In this process internodes along partially myelinated axons were typically not reestablished, while myelin sheaths forming continuous patterns showed remarkable homeostatic resiliences and remyelinated with single axon precision. Understanding these principles is particularly important in the context of multiple sclerosis which extensive cortical demyelination is a major pathological feature and target of remyelination therapies.
Snaidero N, Schifferer M, Mezydlo A, Zalc B, Kerschensteiner M, Misgeld T. Myelin replacement triggered by single-cell demyelination in mouse cortex. Nat Commun. 2020 Sep 29;11(1):4901.

Twin effect overrides disease specific immune signatures
September 2020 – In this study we used our unique setting of the MS TWIN STUDY to explore the peripheral immune signature in collaboration with the UK Münster. In each twin pair, the immune signatures were remarkably similar, pointing to a strong influence of shared genetic and environmental factors. However, when we focused on a subgroup of seemingly healthy cotwins who showed subtle signs of “subclinical neuro-inflammation,” we identified a distinct signature of memory T cells.
Gerdes LA, Janoschka C, Eveslage M, Mannig B, Wirth T, Schulte-Mecklenbeck A, Lauks S, Glau L, Gross CC, Tolosa E, Flierl-Hecht A, Ertl-Wagner B, Barkhof F, Meuth SG, Kümpfel T, Wiendl H, Hohlfeld R, Klotz L. Immune signatures of prodromal multiple sclerosis in monozygotic twins. Proc Natl Acad Sci U S A. 2020 Sep 1;117(35):21546-21556.

Neuronal activity controls circuit formation following spinal cord injury
November 2019 – Here we report on the principles that drive functional circuit remodeling following spinal cord injury. We demonstrate in this paper that functional circuit remodeling critically depends on the selection of appropriate synaptic connections between cortical projection and spinal relay neurons. In particular, we use in this paper a combination of genetic and chemogenetic tools to modulate NMDA receptor (NMDAR) integrity and function, CREB-mediated transcription, and neuronal firing of relay neurons during injury-induced corticospinal remodeling. We show that NMDAR signaling and CREB-mediated transcription maintain nascent corticospinal tract (CST)–relay neuron contacts. These activity-dependent signals act during a defined period of circuit remodeling and do not affect mature or uninjured circuits. Furthermore, chemogenetic modulation of relay neuron activity reveals that the regrowing CST axons select their postsynaptic partners in a competitive manner and that preventing such activity-dependent shaping of corticospinal circuits limits motor recovery after spinal cord injury.
Bradley PM, Denecke CK, Aljovic A, Schmalz A, Kerschensteiner M, Bareyre FM. Corticospinal circuit remodeling after central nervous system injury is dependent on neuronal activity. J Exp Med. 2019 Nov 4;216(11):2503-2514.

Detection of early adaptive immune activation in prodromal MS
November 2019 – The early immunological events that drive MS are still enigmatic. To add a piece to the jigsaw we analyzed CSF samples of a unique selection of our MS TWIN STUDY. Using single-cell RNA sequencing we identified clonally expanded CD8+ T cells, plasmablasts, and, to a lesser extent, CD4+ T cells not only from MS co-twins but also from clinical healthy co-twins with signs of subclinical neuroinflammation as detected on MRI and/or CSF. Strikingly, clonally expanded T cells showed characteristics of activated tissue-resident memory T (TRM) cells and were already detectable in prodromal stages but more pronounced in patients with definite MS. Our data provide evidence for very early concomitant activation of 3 components of the adaptive immune system in MS, with a notable contribution of clonally expanded TRM-like CD8+ cells.
Beltrán E, Gerdes LA, Hansen J, Flierl-Hecht A, Krebs S, Blum H, Ertl-Wagner B, Barkhof F, Kümpfel T, Hohlfeld R, Dornmair K. Early adaptive immune activation detected in monozygotic twins with prodromal multiple sclerosis. J Clin Invest. 2019 Nov 1;129(11):4758-4768.

Circulating autoreactive B cells specific for GAD65
March 2019 – Here we detect GAD-specific B cells that readily differentiate into antibody-producing cells in patients with GAD-antibody-associated neurological disorders. These GAD-specific B cells are as abundant as B cells reactive for common recall antigens. Bone marrow cells represent an additional source of GAD antibodies. The identification of GAD-antibody-producing cells has implications for the selection of cell-specific therapies.
Thaler FS, Thaller AL, Biljecki M, Schuh E, Winklmeier S, Mahler CF, Gerhards R, Völk S, Schnorfeil F, Subklewe M, Hohlfeld R, Kümpfel T, Meinl E. Abundant glutamic acid decarboxylase (GAD)-reactive B cells in gad-antibody-associated neurological disorders. Ann Neurol. 2019 Mar;85(3):448-454. doi: 10.1002/ana.25414. Epub 2019 Jan 28. PMID: 30635933.

Leaky membranes promote axon degeneration in MS model
Februar 2019 – To reveal the mechanisms that drive inflammatory axon degeneration the Kerschensteiner lab has used in vivo calcium imaging in a multiple sclerosis model. Dynamic tracking of individual axons in the inflamed spinal cord shows that cytoplasmic calcium levels determine the choice between axon loss and survival. Calcium can enter the axon through nanoscale ruptures of the axonal plasma membrane that are induced in inflammatory lesions. These results thus identify a unusual axon injury pathway that can contribute to neurodegeneration in multiple sclerosis and may represent a novel target for protective therapy.
Witte ME, Schumacher AM, Mahler CF, Bewersdorf JP, Lehmitz J, Scheiter A, Sánchez P, Williams PR, Griesbeck O, Naumann R, Misgeld T, Kerschensteiner M. Calcium Influx through Plasma-Membrane Nanoruptures Drives Axon Degeneration in a Model of Multiple Sclerosis. Neuron. 2019 Feb 20;101(4):615-624.e5.

DNA methylation signatures are associated with MS phenotype
May 2019 – The modest concordance rate for MS in monozygotic twins strongly argues for involvement of epigenetic factors. To check the contribution of epigenetic modifications we looked at the methylomes of 45 pairs from the MS TWIN STUDY together with our collaborators Nicole Souren and Jörn Walter, Saarland Universtiy. We identified seven MS-associated differentially methylated positions (DMPs) of which we validated two, including a region in the TMEM232 promoter and ZBTB16 enhancer. Not expected but not of less relevance we presented epigenetic biomarkers for current interferon-beta treatment, and that the ZBTB16 DMP is a signature for prior glucocorticoid treatment. Taken together, this study represents an important reference for epigenomic MS studies, identifies new candidate epigenetic markers, and highlights treatment effects and genetic background as major confounders.
Souren NY, Gerdes LA, Lutsik P, Gasparoni G, Beltrán E, Salhab A, Kümpfel T, Weichenhan D, Plass C, Hohlfeld R, Walter J. DNA methylation signatures of monozygotic twins clinically discordant for multiple sclerosis. Nat Commun. 2019 May 7;10(1):2094.

Pathogenic mechanisms of patient antibodies recognizing MOG
August 2018 –Autoantibodies against myelin oligodendrocyte glycoprotein (MOG) can be detected in the blood of a proportion of patients with inflammatory demyelinating diseases of the CNS. We affinity-purified MOG-Abs from the blood of two patients. These anti-MOG Abs were pathogenic upon transfer into rats and we could dissect two different mechanisms by which these MOG-Abs enhanced pathology. First, together with cognate MOG-specific T cells, these Abs enhanced T cell infiltration; Second, together with MBP-specific T cells that strongly breach the blood-brain barrier, these MOG-Abs induced demyelination associated with deposition of C9neo, resembling a multiple sclerosis type II pathology. This suggests that MOG-Abs are similarly pathogenic in patients.
Spadaro M, Winklmeier S, Beltrán E, Macrini C, Höftberger R, Schuh E, Thaler FS, Gerdes LA, Laurent S, Gerhards R, Brändle S, Dornmair K, Breithaupt C, Krumbholz M, Moser M, Krishnamoorthy G, Kamp F, Jenne D, Hohlfeld R, Kümpfel T, Lassmann H, Kawakami N, Meinl E. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann Neurol. 2018 Aug;84(2):315-328.

Shifty phagocytes track the fate of neuroinflammatory lesions
Februar 2018 – Mononuclear phagocytes can both promote and inhibit inflammation. Here the Kerschensteiner lab uses an in vivo imaging approach to follow the evolution of phagocyte phenotypes in neuroinflammatory lesions. By tracking phagocytes over time they can show that individual phagocytes switch their phenotype as lesions move from expansion to resolution. This phenotype shift appears to be initiated by signals derived from the nervous system tissue. Understanding the molecular nature of these signals might thus provide the basis for therapeutic manipulation of phagocyte function in the inflamed CNS.
Locatelli G, Theodorou D, Kendirli A, Jordão MJC, Staszewski O, Phulphagar K, Cantuti-Castelvetri L, Dagkalis A, Bessis A, Simons M, Meissner F, Prinz M, Kerschensteiner M. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat Neurosci. 2018 Sep;21(9):1196-1208.

What´s it got to do with our guts? A possible link between the gut microbiome and MS!
August 2017 – Studies using experimental models have indicated that MS like disease can be triggered in the gut following interactions of brain autoimmune T lymphocytes with local microbiota. In this key paper, we studied the composition of the gut microbiota in our MS TWIN STUDY and in addition transferred human-derived microbiota into transgenic mice expressing a myelin autoantigen-specific T cell receptor. Strikingly, we detected that gut microbiota from MS-affected twins induced CNS-specific autoimmunity at a higher incidence than microbiota from healthy co-twins. Our results offered functional evidence that human microbiome components contribute to CNS-specific autoimmunity and opened up the field for further studies of the gut microbiome in MS.
Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, Kümpfel T, Hohlfeld R, Krishnamoorthy G, Wekerle H. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):10719-10724.

Novel biomarkers for a subset of brain tumors
November 2017 – Previously, we have identified the soluble receptors sTACI and sBCMA and uncovered the biochemical mechanisms of their shedding (Hoffmann et al. J. Immunol. 2015; Laurent et al. Nat commun. 2015;). Here we report in collaboration with Louisa von Baumgarten that sTACI and sBCMA are promising new biomarkers for diagnosis and therapy monitoring in primary central nervous system lymphoma, which constitutes 3-5 % of brain tumors and is highly aggressive.
Thaler FS, Laurent SA, Huber M, Mulazzani M, Dreyling M, Ködel U, Kümpfel T, Straube A, Meinl E, von Baumgarten L. Soluble TACI and soluble BCMA as biomarkers in primary central nervous system lymphoma. Neuro Oncol. 2017 Nov 29;19(12):1618-1627.

Early stages of myelin injury revealed by ultrastructural and dynamic analysis
November 2016 – Damage to oligodendrocytes and their myelin sheaths is a central feature of multiple sclerosis pathology. Here the Kerschensteiner lab investigates how oligodendrocyte damage is initiated in multiple sclerosis (MS) and EAE using in vivo imaging, confocal microscopy as well as electron microscopy. They can show that oligodendrocyte damage spreads centripetally and that the formation of focal myelin outfoldings that they call “myelinosomes” is an early sign of oligodendrocyte damage both in MS and EAE…
Romanelli E, Merkler D, Mezydlo A, Weil MT, Weber MS, Nikić I, Potz S, Meinl E, Matznick FE, Kreutzfeldt M, Ghanem A, Conzelmann KK, Metz I, Brück W, Routh M, Simons M, Bishop D, Misgeld T, Kerschensteiner M. Myelinosome formation represents an early stage of oligodendrocyte damage in multiple sclerosis and its animal model. Nat Commun. 2016 Nov 16;7:13275.

Widespread synapse loss in multiple sclerosis gray matter
January 2016 – How neurodegeneration starts in the brains of progressive multiple sclerosis patients is only incompletely understood. Here the Kerschensteiner lab joined forces with the Merkler lab at the University of Genevan and used confocal microscopy of Golgi-Cox impregnated tissue sections to reconstruct single cortical projection neurons in brain sections from multiple sclerosis and control patients. Their study reveals a widespread and pronounced loss of dendritic spines that occurs independently of cortical demyelination and axon loss and indicates the presence of a primary synaptic pathology in multiple sclerosis…
Jürgens T, Jafari M, Kreutzfeldt M, Bahn E, Brück W, Kerschensteiner M, Merkler D. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain. 2016 Jan;139(Pt 1):39-46.

New immunoregulation and biomarker
June 2015 – Plasma cells produce antibodies that inactivate pathogens, but may also cause autoimmune diseases. Therefore a balanced regulation of plasma cells is essential. We found that the lifespan of plasma cells is regulated through shedding of their survival receptor BCMA by γ-secretase. We have identified the first membrane protein directly shed by γ-secretase. The released part of BCMA reflects the antibody production by plasma cells in the brain of MS patients.
Laurent SA, Hoffmann FS, Kuhn PH, Cheng Q, Chu Y, Schmidt-Supprian M, Hauck SM, Schuh E, Krumbholz M, Rübsamen H, Wanngren J, Khademi M, Olsson T, Alexander T, Hiepe F, Pfister HW, Weber F, Jenne D, Wekerle H, Hohlfeld R, Lichtenthaler SF, Meinl E. γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. 2015 Jun 11;6:7333.

FGF22 regulates circuit formation following spinal cord injury
May 2015 – Following spinal cord injury, transected projections form detour circuits that circumvent the lesion and contribute to functional recovery. The formation of new synaptic contacts is a crucial step of the process, but its molecular regulation is currently not understood. Members of the FGF family can promote synapse formation during nervous system development, suggesting that they might have a similar function in the injured adult CNS. Here, we show that: (i) FGF22 and FGF22 receptors are expressed in the adult nervous system. (ii) FGF22 deficiency or deletion of FGF22 receptors restricts the formation and maturation of new synapses in the injured spinal cord. (iii) Genetic disruption of FGF22 signaling impedes spontaneous functional recovery following spinal cord injury
Jacobi A, Loy K, Schmalz AM, Hellsten M, Umemori H, Kerschensteiner M, Bareyre FM. FGF22 signaling regulates synapse formation during post-injury remodeling of the spinal cord. EMBO J. 2015 May 5;34(9):1231-43.
2025
2024
Todorov-Völgyi, K., González-Gallego, J., Müller, S. A., Beaufort, N., Malik, R., Schifferer, M., Todorov, M. I., Crusius, D., Robinson, S., Schmidt, A., Körbelin, J., Bareyre, F., Ertürk, A., Haass, C., Simons, M., Paquet, D., Lichtenthaler, S. F., & Dichgans, M. (2024). Proteomics of mouse brain endothelium uncovers dysregulation of vesicular transport pathways during aging. Nature aging, 4(4), 595–612.
Gross, C. C., Schulte-Mecklenbeck, A., Steinberg, O. V., Wirth, T., Lauks, S., Bittner, S., Schindler, P., Baranzini, S. E., Groppa, S., Bellmann-Strobl, J., Bünger, N., Chien, C., Dawin, E., Eveslage, M., Fleischer, V., Gonzalez-Escamilla, G., Gisevius, B., Haas, J., Kerschensteiner, M., Kirstein, L., … German Competence Network Multiple Sclerosis (KKNMS) (2024). Multiple sclerosis endophenotypes identified by high-dimensional blood signatures are associated with distinct disease trajectories. Science Translational Medicine, 16(740), eade8560.
Douthwaite C, Tietje C, Ye X, Liebscher S. (2024) Probing cerebellar circuit dysfunction in rodent models of spinocerebellar ataxia by means of in vivo two-photon calcium imaging. STAR protocols. 5(1):102911.
Scekic-Zahirovic J, Benetton C, Brunet A, Ye X, Logunov E, Douchamps V, Megat S, Andry V, Kan VWY, Stuart-Lopez G, Gilet J, Guillot SJ, Dirrig-Grosch S, Gorin C, Trombini M, Dieterle S, Sinniger J, Fischer M, Rene F, Gunes Z, Kessler P, Dupuis L, Pradat PF, Goumon Y, Goutagny R, Marchand-Pauvert V*, Liebscher S*, Rouaux C*. (2024) Cortical hyperexcitability in mouse models and patients with amyotrophic lateral sclerosis is linked to noradrenaline deficiency. Science Translational Medicine. 16(738):eadg3665 (*equal contribution)
2023
Ghezzi P, Lucas R, Mader S, Miossec P, Sacre S. (2023) Editorial: Insights in inflammation: 2022. Front Immunol. 14:1224343.
Teschner VE, Fleck AK, Walter C, Schwarze AS, Eschborn M, Wirth T, Steinberg OV, Schulte-Mecklenbeck A, Lu IN, Herrera-Rivero M, Janoschka C, Lünemann JD, Schwab N, Meyer Zu Hörste G, Varghese J, Gross CC, Pul R, Kleinschnitz C, Mader S, Meinl E, Stoll M, Wiendl H, Klotz L. (2023) Single-cell profiling reveals preferential reduction of memory B cell subsets in cladribine patients that correlates with treatment response. Ther Adv Neurol Disord. 16:17562864231211077.
Ballweg A, Klaus C, Vogler L, Katzdobler S, Wind K, Zatcepin A, Ziegler SI, Secgin B, Eckenweber F, Bohr B, Bernhardt A, Fietzek U, Rauchmann BS, Stoecklein S, Quach S, Beyer L, Scheifele M, Simmet M, Joseph E, Lindner S, Berg I, Koglin N, Mueller A, Stephens AW, Bartenstein P, Tonn JC, Albert NL, Kümpfel T, Kerschensteiner M, Perneczky R, Levin J, Paeger L, Herms J, Brendel M.(2023) [18F]F-DED PET imaging of reactive astrogliosis in neurodegenerative diseases: preclinical proof of concept and first-in-human data. J Neuroinflammation. 20(1):68.
Kolabas ZI, Kuemmerle LB, Perneczky R, Förstera B, Ulukaya S, Ali M, Kapoor S, Bartos LM, Büttner M, Caliskan OS, Rong Z, Mai H, Höher L, Jeridi D, Molbay M, Khalin I, Deligiannis IK, Negwer M, Roberts K, Simats A, Carofiglio O, Todorov MI, Horvath I, Ozturk F, Hummel S, Biechele G, Zatcepin A, Unterrainer M, Gnörich J, Roodselaar J, Shrouder J, Khosravani P, Tast B, Richter L, Díaz-Marugán L, Kaltenecker D, Lux L, Chen Y, Zhao S, Rauchmann BS, Sterr M, Kunze I, Stanic K, Kan VWY, Besson-Girard S, Katzdobler S, Palleis C, Schädler J, Paetzold JC, Liebscher S, Hauser AE, Gokce O, Lickert H, Steinke H, Benakis C, Braun C, Martinez-Jimenez CP, Buerger K, Albert NL, Höglinger G, Levin J, Haass C, Kopczak A, Dichgans M, Havla J, Kümpfel T, Kerschensteiner M, Schifferer M, Simons M, Liesz A, Krahmer N, Bayraktar OA, Franzmeier N, Plesnila N, Erener S, Puelles VG, Delbridge C, Bhatia HS, Hellal F, Elsner M, Bechmann I, Ondruschka B, Brendel M, Theis FJ, Erturk A. (2023) Distinct molecular profiles of skull bone marrow in health and neurological disorders. Cell. 186(17):3706-3725.e29.
Kislinger G, Niemann C, Rodriguez L, Jiang H, Fard MK, Snaidero N, Schumacher AM, Kerschensteiner M, Misgeld T, Schifferer M. (2023) Neurons on tape: Automated Tape Collecting Ultramicrotomy-mediated volume EM for targeting neuropathology. Methods Cell Biol. 177:125-170.
Thomann AS, McQuade CA, Pinjušić K, Kolz A, Schmitz R, Kitamura D, Wekerle H, Peters A. (2023) A B cell-driven EAE mouse model reveals the impact of B cell-derived cytokines on CNS autoimmunity. Proc Natl Acad Sci U S A. 120(47):e2300733120.
Kendirli A, de la Rosa C, Lämmle KF, Eglseer K, Bauer IJ, Kavaka V, Winklmeier S, Zhuo L, Wichmann C, Gerdes LA, Kümpfel T, Dornmair K, Beltrán E, Kerschensteiner M*, Kawakami N* (2023) A genome-wide in vivo CRISPR screen identifies essential regulators of T cell migration to the CNS in a multiple sclerosis model. Nat Neurosci. 26(10):1713-1725.
Tai, Y. H., Engels, D., Locatelli, G., Emmanouilidis, I., Fecher, C., Theodorou, D., Müller, S. A., Licht-Mayer, S., Kreutzfeldt, M., Wagner, I., de Mello, N. P., Gkotzamani, S. N., Trovò, L., Kendirli, A., Aljović, A., Breckwoldt, M. O., Naumann, R., Bareyre, F. M., Perocchi, F., Mahad, D., … Misgeld, T. (2023). Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions. Nature metabolism, 5(8), 1364–1381.
Boldrini, V. O., Mader, S., Kümpfel, T., & Meinl, E. (2023). Ublituximab: A new FDA-approved anti-CD20 mAb for relapsing forms of multiple sclerosis. Mult scler relat disord, 75, 104733.
Fichtner, M. L., Rübsamen, H., Smolle, M., Schaller, J., Feederle, R., Bültmann, A., Kümpfel, T., Schneider, P., Thaler, F. S., & Meinl, E. (2023). Features of Isoforms of Human Soluble TACI. Journal Immunol (Baltimore, Md. : 1950), 211(2), 199–208.
Bauer IJ, Fang P, Lämmle KF, Tyystjärvi S, Alterauge D, Baumjohann D, Yoon H, Korn T, Wekerle H, Kawakami N. (2023) Visualizing the activation of encephalitogenic T cells in the ileal lamina propria by in vivo two-photon imaging. Proc Natl Acad Sci U S A. 120(30):e2302697120.
Engels, D., Mader, S., Förderreuther, S., Reindl, M., Havla, J., Meinl, E., Kümpfel, T., & Gerdes, L. A. (2023). MOG-IgG-Associated Bilateral Optic Neuritis in Temporal Relation to Monkeypox Vaccination. Ann neurol, 93(6), 1216–1217.
Chovsepian A, Empl L, Bareyre FM. Plasticity of callosal neurons in the contralesional cortex following traumatic brain injury. Neural Regen Res. 2023 Jun;18(6):1257-1258.
Mezydlo A, Treiber N, Ullrich Gavilanes EM, Eichenseer K, Ancău M, Wens A, Ares Carral C, Schifferer M, Snaidero N, Misgeld T, Kerschensteiner M. Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron. 2023 Apr 13:S0896-6273(23)00227-1.
Van Steenbergen V, Burattini L, Trumpp M, Fourneau J, Aljović A, Chahin M, Oh H, D'Ambra M, Bareyre FM. Coordinated neurostimulation promotes circuit rewiring and unlocks recovery after spinal cord injury. J Exp Med. 2023 Mar 6;220(3):e20220615.
Mader S, Ho S, Wong HK, Baier S, Winklmeier S, Riemer C, Rübsamen H, Fernandez IM, Gerhards R, Du C, Chuquisana O, Lünemann JD, Lux A, Nimmerjahn F, Bradl M*, Kawakami N*, Meinl E*. Dissection of complement and Fc-receptor-mediated pathomechanisms of autoantibodies to myelin oligodendrocyte glycoprotein. Proc Natl Acad Sci U S A. 2023 Mar 28;120(13):e2300648120.
Thaler FS, Meinl E. Cytotoxic T cells and plasma cells dominate early in temporal lobe epilepsy with GAD antibodies. Brain. 2023 Apr 19;146(4):1231-1233. doi: 10.1093/brain/awad066. Erratum in: Brain. 2023 Apr 17;: PMID: 36864690
Biljecki M, Eisenhut K, Beltrán E, Winklmeier S, Mader S, Thaller A, Eichhorn P, Steininger P, Flierl-Hecht A, Lewerenz J, Kümpfel T, Kerschensteiner M, Meinl E, Thaler FS. Antibodies Against Glutamic Acid Decarboxylase 65 Are Locally Produced in the CSF and Arise During Affinity Maturation. Neurol Neuroimmunol Neuroinflamm. 2023 Feb 23;10(3):e200090.
Spatola M, Chuquisana O, Jung W, Lopez JA, Wendel EM, Ramanathan S, Keller CW, Hahn T, Meinl E, Reindl M, Dale RC, Wiendl H, Lauffenburger DA, Rostásy K, Brilot F, Alter G, Lünemann JD. Humoral signatures of MOG-antibody-associated disease track with age and disease activity. Cell Rep Med. 2023 Feb 21;4(2):100913
Gernert JA, Wicklein R, Hemmer B, Kümpfel T, Knier B, Havla J. Peripapillary hyper-reflective ovoid mass-like structures (PHOMS) in AQP4-IgG-positive neuromyelitis optica spectrum disease (NMOSD) and MOG-IgG-associated disease (MOGAD). J Neurol. 2023 Feb;270(2):1135-1140.
Ho S, Oswald E, Wong HK, Vural A, Yilmaz V, Tüzün E, Türkoğlu R, Straub T, Meinl I, Thaler F, Kümpfel T, Meinl E*, Mader S*.(2023) Ocrelizumab Treatment Modulates B-Cell Regulating Factors in Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm. 10(2):e200083.
Aljović A, Jacobi A, Marcantoni M, Kagerer F, Loy K, Kendirli A, Bräutigam J, Fabbio L, Van Steenbergen V, Pleśniar K, Kerschensteiner M, Bareyre FM. Synaptogenic gene therapy with FGF22 improves circuit plasticity and functional recovery following spinal cord injury. EMBO Mol Med. 2023 Jan 5:e16111.
2022
Zambusi A, Novoselc KT, Hutten S, Kalpazidou S, Koupourtidou C, Schieweck R, Aschenbroich S, Silva L, Yazgili AS, van Bebber F, Schmid B, Möller G, Tritscher C, Stigloher C, Delbridge C, Sirko S, Günes ZI, Liebscher S, Schlegel J, Aliee H, Theis F, Meiners S, Kiebler M, Dormann D, Ninkovic J. TDP-43 condensates and lipid droplets regulate the reactivity of microglia and regeneration after traumatic brain injury. Nat Neurosci. 2022 Dec;25(12):1608-1625.
Kenney R, Liu M, Hasanaj L, Joseph B, Al-Hassan AA, Balk L, Behbehani R, Brandt AU, Calabresi PA, Frohman EM, Frohman T, Havla J, Hemmer B, Jiang H, Knier B, Korn T, Leocani L, Martínez-Lapiscina EH, Papadopoulou A, Paul F, Petzold A, Pisa M, Villoslada P, Zimmermann H, Ishikawa H, Schuman JS, Wollstein G, Chen Y, Saidha S, Thorpe LE, Galetta SL, Balcer LJ; IMSVISUAL Consortium. Normative Data and Conversion Equation for Spectral-Domain Optical Coherence Tomography in an International Healthy Control Cohort. J Neuroophthalmol. 2022 Dec 1;42(4):442-453.
Gernert JA, Zimmermann H, Oswald E, Christmann T, Kümpfel T, Havla J. Clinical onset of CNS demyelinating disease after COVID-19 vaccination: denovo disease? Mult Scler Relat Disord. 2022 Nov;67:104175.
Appeltshauser L, Junghof H, Messinger J, Linke J, Haarmann A, Ayzenberg I, Baka P, Dorst J, Fisse AL, Grüter T, Hauschildt V, Jörk A, Leypoldt F, Mäurer M, Meinl E, Michels S, Motte J, Pitarokoili K, Stettner M, Villmann C, Weihrauch M, Welte GS, Zerr I, Heinze KG, Sommer C, Doppler K. Anti-pan-neurofascin antibodies induce subclass-related complement activation and nodo-paranodal damage. Brain. 2022 Nov 8:awac418.
Tabansky I*, Tanaka A*, Wang J*, Zhang G*, Dujmovic I*, Mader S*,Jeganathan V, DeAngelis T, Funaro M, Harel A, Messina Mark, Shabbir M, Nursey V, DeGouvia W,Laurent M, Blitz K, Jindra P, Gudesblatt M, Regeneron Genetics Center, King A, Drulovic J, Yunis E, Brusic V, Shen Y, Keskin D, Najja S and Stern J N. H. Rare variants and HLA haplotypes associated in patients with neuromyelitis optica spectrum disorders. Front Immunol . 2022 Oct 4;13:900605. (* co-first authors).
Lotz-Havla AS, Katzdobler S, Nuscher B, Weiß K, Levin J, Havla J, Maier EM. Serum glial fibrillary acidic protein and neurofilament light chain in patients with early treated phenylketonuria. Front Neurol. 2022 Sep 29;13:1011470.
Schneider J, Weigel J, Wittmann MT, Svehla P, Ehrt S, Zheng F, Elmzzahi T, Karpf J, Paniagua-Herranz L, Basak O, Ekici A, Reis A, Alzheimer C, Ortega de la O F, Liebscher S, Beckervordersandforth R. Astrogenesis in the murine dentate gyrus is a life-long and dynamic process. EMBO J. 2022 Jun 1;41(11):e110409.
Mader S, Kümpfel T, Meinl E. Pathomechanisms in demyelination and astrocytopathy: autoantibodies to AQP4, MOG, GFAP, GRP78 and beyond. Curr Opin Neurol. 2022 Jun 1;35(3):427-435.
Unterrainer M, Mahler C, Schumacher AM, Ruf V, Blum B, Quach S, Brendel M, Rupprecht R, Bartenstein P, Kerschensteiner M, Kümpfel T, Albert NL. Amino Acid Uptake, Glucose Metabolism, and Neuroinflammation in John Cunningham Virus Associated Progressive Multifocal Leukoencephalopathy. Clin Nucl Med. 2022 Jun 1;47(6):543-544.
Ingelfinger F, Beltrán E, Gerdes LA, Becher B. Single-cell multiomics in neuroinflammation. Curr Opin Immunol. 2022 Jun;76:102180.
Fourneau J, Bareyre FM. Semaphorin7A: its role in the control of serotonergic circuits and functional recovery following spinal cord injury. Neural Regen Res. 2022 May;17(5):959-962.
Preisendörfer S, Ishikawa Y, Hennen E, Winklmeier S, Schupp JC, Knüppel L, Fernandez IE, Binzenhöfer L, Flatley A, Juan-Guardela BM, Ruppert C, Guenther A, Frankenberger M, Hatz RA, Kneidinger N, Behr J, Feederle R, Schepers A, Hilgendorff A, Kaminski N, Meinl E, Bächinger HP, Eickelberg O, Staab-Weijnitz CA. FK506-Binding Protein 11 Is a Novel Plasma Cell-Specific Antibody Folding Catalyst with Increased Expression in Idiopathic Pulmonary Fibrosis. Cells. 2022 Apr 14;11(8):1341.
Meinl E. Multiple Sklerose und Epstein-Barr Virus: gesicherte Erkenntnisse und offene Fragen. Neurodiem 2022
Schneider-Hohendorf T*, Gerdes LA*, Pignolet B, Gittelman R, Ostkamp P, Rubelt F, Raposo C, Tackenberg B, Riepenhausen M, Janoschka C, Wünsch C, Bucciarelli F, Flierl-Hecht A, Beltrán E, Kümpfel T, Anslinger K, Gross CC, Chapman H, Kaplan I, Brassat D, Wekerle H, Kerschensteiner M, Klotz L, Lünemann JD, Hohlfeld R, Liblau R, Wiendl H, Schwab N. Broader Epstein-Barr virus-specific T cell receptor repertoire in patients with multiple sclerosis.J Exp Med. 2022;219(11):e20220650.
Ingelfinger F, Gerdes LA, Kavaka V, Krishnarajah S, Friebel E, Galli E, Zwicky P, Furrer R, Peukert C, Dutertre CA, Eglseer KM, Ginhoux F, Flierl-Hecht A, Kümpfel T, De Feo D, Schreiner B, Mundt S, Kerschensteiner M, Hohlfeld R, Beltrán E, Becher B. Twin study reveals non-heritable immune perturbations in multiple sclerosis. Nature. 2022 Mar;603(7899):152-158.
Riederer I, Mühlau M, Wiestler B, Bender B, Hempel JM, Kowarik M, Huber T, Zimmer C, Andrisan T, Patzig M, Zimmermann H, Havla J, Berlis A, Behrens L, Beer M, Dietrich J, Sollmann N, Kirschke JS. Structured Reporting in Multiple Sclerosis - Consensus-Based Reporting Templates for Magnetic Resonance Imaging of the Brain and Spinal Cord. Rofo. 2022 Jul 29.
Oertel FC*, Sotirchos ES*, Zimmermann HG, Motamedi S, Specovius S, Asseyer ES, Chien C, Cook L, Vasileiou E, Filippatou A, Calabresi PA, Saidha S, Pandit L, D'Cunha A, Outteryck O, Zéphir H, Pittock S, Flanagan EP, Bhatti MT, Rommer PS, Bsteh G, Zrzavy T, Kuempfel T, Aktas O, Ringelstein M, Albrecht P, Ayzenberg I, Pakeerathan T, Knier B, Aly L, Asgari N, Soelberg K, Marignier R, Tilikete CF, Calvo AC, Villoslada P, Sanchez-Dalmau B, Martinez-Lapiscina EH, Llufriu S, Green AJ, Yeaman MR, Smith TJ, Brandt AU, Chen J*, Paul F*, Havla J*; with the GJCF International Clinical Consortium for NMOSD and the CROCTINO study group. Longitudinal retinal changes in MOGAD. Ann Neurol. 2022 Sep;92(3):476-485.
Laura Empl, Alexandra Chovsepian, Maryam Chahin, Wing Yin Vanessa Kan, Julie Fourneau, Valérie Van Steenbergen, Sanofer Weidinger, Maite Marcantoni, Alexander Ghanem, Peter Bradley, Karl Klaus Conzelmann, Ruiyao Cai, Alireza Ghasemigharagoz, Ali Ertürk, Ingrid Wagner, Mario Kreutzfeldt, Doron Merkler, Sabine Liebscher & Florence M. Bareyre. Selective plasticity of callosal neurons in the adult contralesional cortex following murine traumatic brain injury. Nat Commun 13, 2659 (2022).
Aljovic A, Zhao S, Chahin M, de la Rosa C, Van Steenbergen V, Kerschensteiner M, Bareyre FM. A deep learning-based toolbox for Automated Limb Motion Analysis (ALMA) in murine models of neurological disorders. Commun Biol. 2022 Feb 15;5(1):131.
Havla J, Hohlfeld R. Antibody Therapies for Progressive Multiple Sclerosis and for Promoting Repair. Neurotherapeutics. 2022 Apr;19(3):774-784.
Mader S, Brimberg L, Vo A, Strohl JJ, Crawford JM, Bonnin A, Carrión J, Campbell D, Huerta TS, La Bella A, Berlin R, Dewey SL, Hellman M, Eidelberg D, Dujmovic I, Drulovic J, Bennett JL, Volpe BT, Huerta PT, Diamond B. In utero exposure to maternal anti-aquaporin-4 antibodies alters brain vasculature and neural dynamics in male mouse offspring. Sci Transl Med. 2022, 20;14(641):eabe9726.
Hümmert MW, Schöppe LM, Bellmann-Strobl J, Siebert N, Paul F, Duchow A, Pellkofer H, Kümpfel T, Havla J, Jarius S, Wildemann B, Berthele A, Bergh FT, Pawlitzki M, Klotz L, Kleiter I, Stangel M, Gingele S, Weber MS, Faiss JH, Pul R, Walter A, Zettl UK, Senel M, Stellmann JP, Häußler V, Hellwig K, Ayzenberg I, Aktas O, Ringelstein M, Schreiber-Katz O, Trebst C; Neuromyelitis Optica Study Group (NEMOS). Costs and Health-Related Quality of Life in Patients With NMO Spectrum Disorders and MOG-Antibody-Associated Disease: CHANCENMO Study. Neurology. 2022 Mar 15;98(11):e1184-e1196.
Eisenhut K, Buchka S, Eichhorn P, Meier H, Albashiti F, Mansmann U, Schlüter M, Havla J, Kümpfel T. SARS-CoV-2 antibody seroprevalence in a large neuroimmunological patient cohort. J Neurol. 2022 Mar;269(3):1133-1137.
Lu A, Zimmermann HG, Specovius S, Motamedi S, Chien C, Bereuter C, Lana-Peixoto MA, Fontenelle MA, Ashtari F, Kafieh R, Dehghani A, Pourazizi M, Pandit L, D'Cunha A, Kim HJ, Hyun JW, Jung SK, Leocani L, Pisa M, Radaelli M, Siritho S, May EF, Tongco C, De Sèze J, Senger T, Palace J, Roca-Fernández A, Leite MI, Sharma SM, Stiebel-Kalish H, Asgari N, Soelberg KK, Martinez-Lapiscina EH, Havla J, Mao-Draayer Y, Rimler Z, Reid A, Marignier R, Cobo-Calvo A, Altintas A, Tanriverdi U, Yildirim R, Aktas O, Ringelstein M, Albrecht P, Tavares IM, Bichuetti DB, Jacob A, Huda S, Soto de Castillo I, Petzold A, Green AJ, Yeaman MR, Smith TJ, Cook L, Paul F, Brandt AU, Oertel FC; GJCF International Clinical Consortium for NMOSD. Astrocytic outer retinal layer thinning is not a feature in AQP4-IgG seropositive neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2022 Feb;93(2):188-195.
Kerschensteiner M, Misgeld T. A less painful transfer of power. Neuron. 2022 Feb 16;110(4):559-561.
Winklmeier S, Eisenhut K, Taskin D, Rübsamen H, Gerhards R, Schneider C, Wratil PR, Stern M, Eichhorn P, Keppler OT, Klein M, Mader S, Kümpfel T, Meinl E. Persistence of functional memory B cells recognizing SARS-CoV-2 variants despite loss of specific IgG. iScience. 2022 Jan 21;25(1):103659.
Ingelfinger F*, Gerdes LA*, Kavaka V, Krishnarajah S, Friebel E, Galli E, Zwicky P, Furrer R, Peukert C, Dutertre CA, Eglseer KM, Ginhoux F, Flierl-Hecht A, Kümpfel T, De Feo D, Schreiner B, Mundt S, Kerschensteiner M, Hohlfeld R, Beltrán E#, Becher B#. Twin study reveals non-heritable immune perturbations in multiple sclerosis. Nature. 2022. 603(7899):152-158.
Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol. 2022 Jan;269(1):55-58.
2021
Korzhova V., Marinković P., Njavro, J.R., Goltstein P.M., Sun F., Tahirovic S., Herms J., Liebscher S. Long-term dynamics of aberrant neuronal activity in Alzheimer’s disease transgenic mice. Commun Biol. 2021;4(1):1368.
Behrens G, Edelmann SL, Raj T, Kronbeck N, Monecke T, Davydova E, Wong EH, Kifinger L, Giesert F, Kirmaier ME, Hohn C, de Jonge LS, Pisfil MG, Fu M, Theurich S, Feske S, Kawakami N, Wurst W, Niessing D, Heissmeyer V. Disrupting Roquin-1 interaction with Regnase-1 induces autoimmunity and enhances antitumor responses. Nat Immunol. 2021 Dec;22(12):1563-1576.
Asseyer S, Henke E, Trebst C, Hümmert MW, Wildemann B, Jarius S, Ringelstein M, Aktas O, Pawlitzki M, Korsen M, Klotz L, Siebert N, Ruprecht K, Bellmann-Strobl J, Wernecke KD, Häußler V, Havla J, Gahlen A, Gold R, Paul F, Kleiter I, Ayzenberg I; Neuromyelitis Optica Study Group. Pain, depression, and quality of life in adults with MOG-antibody-associated disease. Eur J Neurol. 2021 May;28(5):1645-1658.
Stangel M, Becker V, Elias-Hamp B, Havla J, Grothe C, Pul R, Rau D, Richter S, Schmidt S. Oral pulsed therapy of relapsing multiple sclerosis with cladribine tablets - expert opinion on issues in clinical practice. Mult Scler Relat Disord. 2021 Sep;54:103075.
Lana-Peixoto MA, Fontenelle MA, Ashtari F, Kafieh R, Dehghani A, Pourazizi M, Pandit L, D'Cunha A, Kim HJ, Hyun JW, Jung SK, Leocani L, Pisa M, Radaelli M, Siritho S, May EF, Tongco C, De Sèze J, Senger T, Palace J, Roca-Fernández A, Leite MI, Sharma SM, Stiebel-Kalish H, Asgari N, Soelberg KK, Martinez-Lapiscina EH, Havla J, Mao-Draayer Y, Rimler Z, Reid A, Marignier R, Cobo-Calvo A, Altintas A, Tanriverdi U, Yildirim R, Aktas O, Ringelstein M, Albrecht P, Tavares IM, Bichuetti DB, Jacob A, Huda S, Soto de Castillo I, Petzold A, Green AJ, Yeaman MR, Smith TJ, Cook L, Paul F, Brandt AU, Oertel FC; GJCF International Clinical Consortium for NMOSD. Astrocytic outer retinal layer thinning is not a feature in AQP4-IgG seropositive neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2021 Oct 28:jnnp-2021-327412.
Ostkamp P, Salmen A, Pignolet B, Görlich D, Andlauer TFM, Schulte-Mecklenbeck A, Gonzalez-Escamilla G, Bucciarelli F, Gennero I, Breuer J, Antony G, Schneider-Hohendorf T, Mykicki N, Bayas A, Then Bergh F, Bittner S, Hartung HP, Friese MA, Linker RA, Luessi F, Lehmann-Horn K, Mühlau M, Paul F, Stangel M, Tackenberg B, Tumani H, Warnke C, Weber F, Wildemann B, Zettl UK, Ziemann U, Müller-Myhsok B, Kümpfel T, Klotz L, Meuth SG, Zipp F, Hemmer B, Hohlfeld R, Brassat D, Gold R, Gross CC, Lukas C, Groppa S, Loser K, Wiendl H, Schwab N; German Competence Network Multiple Sclerosis (KKNMS) and the BIONAT Network. Sunlight exposure exerts immunomodulatory effects to reduce multiple sclerosis severity. Proc Natl Acad Sci U S A. 2021 Jan 5;118(1):e2018457118.
Pellkofer HL, Kümpfel T. Schmerzen bei multipler Sklerose und Neuromyelitis-optica-Spektrum-Erkrankungen [Pain in multiple sclerosis and neuromyelitis optica spectrum disorders]. Schmerz. 2021 Jun;35(3):211-222.
Havla J, Pakeerathan T, Schwake C, Bennett JL, Kleiter I, Felipe-Rucián A, Joachim SC, Lotz-Havla AS, Kümpfel T, Krumbholz M, Wendel EM, Reindl M, Thiels C, Lücke T, Hellwig K, Gold R, Rostasy K, Ayzenberg I. Age-dependent favorable visual recovery despite significant retinal atrophy in pediatric MOGAD: how much retina do you really need to see well? J Neuroinflammation. 2021 May 29;18(1):121.
Ayzenberg I, Richter D, Henke E, Asseyer S, Paul F, Trebst C, Hümmert MW, Havla J, Kümpfel T, Ringelstein M, Aktas O, Wildemann B, Jarius S, Häußler V, Stellmann JP, Senel M, Klotz L, Pellkofer HL, Weber MS, Pawlitzki M, Rommer PS, Berthele A, Wernecke KD, Hellwig K, Gold R, Kleiter I; NEMOS (Neuromyelitis Optica Study Group). Pain, Depression, and Quality of Life in Neuromyelitis Optica Spectrum Disorder: A Cross-Sectional Study of 166 AQP4 Antibody-Seropositive Patients. Neurol Neuroimmunol Neuroinflamm. 2021 Apr 20;8(3):e985.
Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol. 2021 Jun 11:1–4.
Schanda K, Peschl P, Lerch M, Seebacher B, Mindorf S, Ritter N, Probst M, Hegen H, Di Pauli F, Wendel EM, Lechner C, Baumann M, Mariotto S, Ferrari S, Saiz A, Farrell M, Leite MIS, Irani SR, Palace J, Lutterotti A, Kümpfel T, Vukusic S, Marignier R, Waters P, Rostasy K, Berger T, Probst C, Höftberger R, Reindl M. Differential Binding of Autoantibodies to MOG Isoforms in Inflammatory Demyelinating Diseases. Neurol Neuroimmunol Neuroinflamm. 2021 Jun 15;8(5):e1027.
Wicklein R, Wauschkuhn J, Giglhuber K, Kümpfel T, Hemmer B, Havla J, Knier B. Association of peripapillary hyper-reflective ovoid masslike structures and disease duration in primary progressive multiple sclerosis. Eur J Neurol. 2021 Dec;28(12).
Eisenhut K, Buchka S, Eichhorn P, Meier H, Albashiti F, Mansmann U, Schlüter M, Havla J, Kümpfel T. SARS-CoV-2 antibody seroprevalence in a large neuroimmunological patient cohort. J Neurol. 2021 Oct 5:1–5.
Dürr M, Nissen G, Sühs KW, Schwenkenbecher P, Geis C, Ringelstein M, Hartung HP, Friese MA, Kaufmann M, Malter MP, Madlener M, Thaler FS, Kümpfel T, Senel M, Häusler MG, Schneider H, Bergh FT, Kellinghaus C, Zettl UK, Wandinger KP, Melzer N, Gross CC, Lange P, Dreyhaupt J, Tumani H, Leypoldt F, Lewerenz J; German Network for Research on Autoimmune Encephalitis. CSF Findings in Acute NMDAR and LGI1 Antibody-Associated Autoimmune Encephalitis. Neurol Neuroimmunol Neuroinflamm. 2021 Oct 25;8(6):e1086.
Graf J, Leussink VI, Soncin G, Lepka K, Meinl I, Kümpfel T, Meuth SG, Hartung HP, Havla J, Aktas O, Albrecht P. Relapse-independent multiple sclerosis progression under natalizumab. Brain Commun. 2021 Oct 9;3(4):fcab229.
Ringelstein M, Ayzenberg I, Lindenblatt G, Fischer K, Gahlen A, Novi G, Hayward-Könnecke H, Schippling S, Rommer PS, Kornek B, Zrzavy T, Biotti D, Ciron J, Audoin B, Berthele A, Giglhuber K, Zephir H, Kümpfel T, Berger R, Röther J, Häußler V, Stellmann JP, Whittam D, Jacob A, Kraemer M, Gueguen A, Deschamps R, Bayas A, Hümmert MW, Trebst C, Haarmann A, Jarius S, Wildemann B, Grothe M, Siebert N, Ruprecht K, Paul F, Collongues N, Marignier R, Levy M, Karenfort M, Deppe M, Albrecht P, Hellwig K, Gold R, Hartung HP, Meuth SG, Kleiter I, Aktas O; Neuromyelitis Optica Study Group (NEMOS). Interleukin-6 Receptor Blockade in Treatment-Refractory MOG-IgG-Associated Disease and Neuromyelitis Optica Spectrum Disorders. Neurol Neuroimmunol Neuroinflamm. 2021 Nov 16;9(1):e1100.
Nishri Y, Fainstein N, Goldfarb S, Hampton D, Macrini C, Meinl E, Chandran S, Ben-Hur T. Modeling compartmentalized chronic immune-mediated demyelinating CNS disease in the Biozzi ABH mouse. J. Neuroimmunol. 2021, 356:577582.
Schlüter M, Oswald E, Winklmeier S, Meinl I, Havla J, Eichhorn P, Meinl E*, Kümpfel T*.Effects of Natalizumab Therapy on Intrathecal Immunoglobulin G Production Indicate Targeting of Plasmablasts. Neurol Neuroimmunol Neuroinflamm. 2021 Jul 1;8(5):e1030.(* co-senior author)
Meinl E, Hohlfeld R. CD20+ T Cells as Pathogenic Players and Therapeutic Targets in MS. Ann Neurol. 2021 Nov;90(5):722-724.
Meinl E, Krumbholz M. Endogenous soluble receptors sBCMA and sTACI: biomarker, immunoregulator and hurdle for therapy in multiple myeloma. Curr Opin Immunol. 2021 Aug;71:117-123.
Marignier R, Hacohen Y, Cobo-Calvo A, Pröbstel AK, Aktas O, Alexopoulos H, Amato MP, Asgari N, Banwell B, Bennett J, Brilot F, Capobianco M, Chitnis T, Ciccarelli O, Deiva K, De Sèze J, Fujihara K, Jacob A, Kim HJ, Kleiter I, Lassmann H, Leite MI, Linington C, Meinl E, Palace J, Paul F, Petzold A, Pittock S, Reindl M, Sato DK, Selmaj K, Siva A, Stankoff B, Tintore M, Traboulsee A, Waters P, Waubant E, Weinshenker B, Derfuss T, Vukusic S, Hemmer B. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021 Sep;20(9):762-772.
Penkert H, Lauber C, Gerl MJ, Klose C, Damm M, Fitzner D, Flierl-Hecht A, Kümpfel T, Kerschensteiner M, Hohlfeld R, Gerdes LA, Simons M. Plasma lipidomics of monozygotic twins discordant for multiple sclerosis. Ann Clin Transl Neurol. 2020 Dec;7(12):2461-2466.
Mahler C, Schumacher AM, Unterrainer M, Kaiser L, Höllbacher T, Lindner S, Havla J, Ertl-Wagner B, Patzig M, Seelos K, Neitzel J, Mäurer M, Krumbholz M, Metz I, Brück W, Stadelmann C, Merkler D, Gass A, Milenkovic V, Bartenstein P, Albert NL, Kümpfel T, Kerschensteiner M. TSPO PET imaging of natalizumab-associated progressive multifocal leukoencephalopathy. Brain. 2021 Oct 22;144(9):2683-2695.
Schifferer M, Snaidero N, Djannatian M, Kerschensteiner M, Misgeld T. Niwaki Instead of Random Forests: Targeted Serial Sectioning Scanning Electron Microscopy With Reimaging Capabilities for Exploring Central Nervous System Cell Biology and Pathology. Front Neuroanat. 2021 Oct 13;15:732506.
Brändle SM, Cerina M, Weber S, Held K, Menke AF, Alcalá C, Gebert D, Herrmann AM, Pellkofer H, Gerdes LA, Bittner S, Leypoldt F, Teegen B, Komorowski L, Kümpfel T, Hohlfeld R, Meuth SG, Casanova B, Melzer N, Beltrán E, Dornmair K. Cross-reactivity of a pathogenic autoantibody to a tumor antigen in GABAA receptor encephalitis. Proc Natl Acad Sci U S A. 2021 Mar 2;118(9):e1916337118.
Mortazavi M, Hizarci Ö, Gerdes LA, Havla J, Kümpfel T, Hohlfeld R, Stöcklein S, Keeser D, Ertl-Wagner B. Multiple sclerosis and subclinical neuropathology in healthy individuals with familial risk: A scoping review of MRI studies. Neuroimage Clin. 2021;31:102734.
Hiltensperger M, Beltrán E, Kant R, Tyystjärvi S, Lepennetier G, Domínguez Moreno H, Bauer IJ, Grassmann S, Jarosch S, Schober K, Buchholz VR, Kenet S, Gasperi C, Öllinger R, Rad R, Muschaweckh A, Sie C, Aly L, Knier B, Garg G, Afzali AM, Gerdes LA, Kümpfel T, Franzenburg S, Kawakami N, Hemmer B, Busch DH, Misgeld T, Dornmair K, Korn T. Skin and gut imprinted helper T cell subsets exhibit distinct functional phenotypes in central nervous system autoimmunity. Nat Immunol. 2021 Jul;22(7):880-892.
Hartlehnert M, Börsch AL, Li X, Burmeister M, Gerwien H, Schafflick D, Heming M, Lu IN, Narayanan V, Strecker JK, Kolz A, Peters A, Wu GF, Wiendl H, Sorokin L, Meyer Zu Horste G. Bcl6 controls meningeal Th17-B cell interaction in murine neuroinflammation. Proc Natl Acad Sci U S A. 2021 Sep 7;118(36):e2023174118.
Hohlfeld R, Beltran E, Gerdes LA, Dornmair K. Tissue-resident CD8+ memory T cells in multiple sclerosis. Brain. 2021 Feb 12;144(1):e7.
Blumstock S, Sun F, Klaus C, Marinkovic P, Sgobio C, Paeger L, Liebscher S* & Herms J*. Cortical circuit dysfunction in a mouse model of alpha-synucleinopathy in vivo. Brain Communications 2021,3(4), fcab273 (* co-senoir author).
Korzhova V, Marinkovic P, Rudan Njavro J, Goltstein PM, Sun F, Tahirovic S, Herms J, Liebscher S. Long-term dynamics of aberrant neuronal activity in awake Alzheimer’s disease transgenic mice. Communications Biology 2021, 4:1368.
Thaler FS, Zimmermann L, Kammermeier S, Strippel C, Ringelstein M, Kraft A, Sühs K-W,Wickel J, Geis C, Markewitz R, Urbanek C, Sommer C, Doppler K, Penner L, Lewerenz J, Rößling R, Finke C, Prüss H, Melzer N, Wandinger K-P, Leypoldt F, Kümpfel T. Rituximab treatment and long-term outcome of patients with autoimmune encephalitis: real-world evidence from the GENERATE registry. Neurol Neuroimmunol Neuroinflamm. 2021 Oct 1;8(6):e1088.
Schwenkenbecher P, Skripuletz T, Lange P, Dürr M, Konen F, Möhn N, Ringelstein M, Menge T, Friese MA, Melzer N, Malter MP, Häusler M, Thaler FS, Stangel M, Lewerenz J, Sühs K-W. Intrathecal antibody production against Epstein-Barr, herpes simplex, and other neurotropic viruses in autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 2021 Aug 24;8(6):e1062
Gaig C, Compta Y, Heidbreder A, Marti MJ, Titulaer MJ, Crijnen Y, Högl B, Lewerenz J, Erro ME, Garcia-Monco JC, Nigro P, Tambasco N, Patalong-Ogiewa M, Erdler M, Macher S, Berger-Sieczkowski E, Höftberger R, Geis C, Hutterer M, Milán-Tomás A, Martin-Bastida A, Lopez Manzanares L, Quintas S, Höglinger GU, Möhn N, Schoeberl F, Thaler FS, Asioli GM, Provini F, Plazzi G, Berganzo K, Blaabjerg M, Brüggemann N, Farias T, Ng CF, Giordana C, Herrero-San Martín A, Huebra L, Kotschet K, Liendl H, Montojo T, Morata C, Perez Perez J, Puertas I, Seifert-Held T, Seitz C, Mistieri Simabukuro M, Tellez N, Villacieros-Álvarez J, Willekens B, Sabater L, Iranzo A, Santamaria Cano J, Dalmau J, Graus F. Frequency and Characterization of Movement Disorders in Anti-IgLON5 Disease. Neurology. 2021 Aug 11;10.1212.
Heinz Steffens, Alexander C Mott, Siyuan Li, Waja Wegner, Pavel Švehla, Vanessa W. Y. Kan, Fred Wolf, Sabine Liebscher* and Katrin I. Willig*: Stable but not rigid: Chronic in vivo STED nanoscopy reveals extensive remodeling of spines, indicating multiple drivers of plasticity. Science Adv 2021. (* co-senior author)
Loy K, Fourneau J, Meng N, Denecke C, Locatelli G, Bareyre FM. Semaphorin 7A restricts serotonergic innervation and ensures recovery after spinal cord injury. Cell Mol Life Sci. 2021 Mar;78(6):2911-2927.
Van Steenbergen V, Bareyre FM. Chemogenetic approaches to unravel circuit wiring and related behavior after spinal cord injury. Exp Neurol. 2021 Nov;345:113839.
Steffens H, Mott AC, Li S, Wegner W, Svehla P, Kan WYV, Wolf F, Liebscher S# & Willig KI#. Stable but not rigid: Chronic in vivo STED nanoscopy reveals extensive remodeling of spines, indicating multiple drivers of plasticity. Science Adv 2021;7(24) # equal contr
Jelena Scekic-Zahirovic*, Inmaculada Sanjuan-Ruiz*, Vanessa Kan, Salim Megat, Pierre De Rossi, Stéphane Dieterlé, Raphaelle Cassel, Marguerite Jamet, Pascal Kessler, Diana Wiesner, Laura Tzeplaeff, Valérie Demais, Sonu Sahadevan, Katharina M. Hembach, Hans-Peter Muller, Gina Picchiarelli, Nibha Mishra, Stefano Antonucci, Sylvie Dirrig-Grosch, Jan Kassubek, Volker Rasche, Albert Ludolph, Anne-Laurence Boutillier, Francesco Roselli, Magdalini Polymenidou, Clotilde Lagier-Tourenne, Sabine Liebscher# and Luc Dupuis#, Cytoplasmic FUS triggers early behavioral alterations linked to cortical neuronal hyperactivity and inhibitory synaptic defects, Nature Communications, 2021,12:3028
Macrini C, Gerhards R, Winklmeier S, Bergmann L, Mader S, Spadaro M, Vural A, Smolle M, Hohlfeld R, Kümpfel T, Lichtenthaler S, Franquelim H, Jenne D, Meinl E. Features of MOG required for recognition by patients with MOG-antibody-associated disorders. 2021, Brain, 2021 Sep 4;144(8):2375-2389.
Hohlfeld R, Beltran E, Gerdes LA, Dornmair K. Tissue-resident CD8+ memory T cells in multiple sclerosis. Brain. 2021 Feb 12;144(1):e7.
Beltrán E, Paunovic M, Gebert D, Cesur E, Jeitler M, Höftberger R, Malotka J, Mader S, Kawakami N, Meinl E, Bradl M, Dornmair K, Lassmann H. Archeological neuroimmunology: resurrection of a pathogenic immune response from a historical case sheds light on human autoimmune encephalomyelitis and multiple sclerosis. Acta Neuropathol. 2021 Jan;141(1):67-83.
Jafari M, Schumacher AM, Snaidero N, Ullrich Gavilanes EM, Neziraj T, Kocsis-Jutka V, Engels D, Jürgens T, Wagner I, Weidinger JDF, Schmidt SS, Beltrán E, Hagan N, Woodworth L, Ofengeim D, Gans J, Wolf F, Kreutzfeldt M, Portugues R, Merkler D, Misgeld T, Kerschensteiner M. Phagocyte-mediated synapse removal in cortical neuroinflammation is promoted by local calcium accumulation. Nat Neurosci. 2021 Jan 25. doi: 10.1038/s41593-020-00780-7.2021 Mar;24(3):355-367.
2020
Mulazzani E, Zolyniak N, Noe E, Mulazzani M, Azad SC, Kümpfel T, Kraft E. Clinical and psychological phenomenology of pain in autoinflammatory diseases. BMC Rheumatol. 2020 Dec 18;4(1):71.
Kislinger G, Gnägi H, Kerschensteiner M, Simons M, Misgeld T, Schifferer M. ATUM-FIB microscopy for targeting and multiscale imaging of rare events in mouse cortex. STAR Protoc. 2020 Dec 16;1(3):100232.
Gerhards R, Pfeffer LK, Lorenz J, Starost L, Nowack L, Thaler FS, Schlüter M, Rübsamen H, Macrini C, Winklmeier S, Mader S, Bronge M, Grönlund H, Feederle R, Hsia HE, Lichtenthaler SF, Merl-Pham J, Hauck SM, Kuhlmann T, Bauer IJ, Beltran E, Gerdes LA, Mezydlo A, Bar-Or A, Banwell B, Khademi M, Olsson T, Hohlfeld R, Lassmann H, Kümpfel T, Kawakami N, Meinl E. Oligodendrocyte myelin glycoprotein as a novel target for pathogenic autoimmunity in the CNS. Acta Neuropathol Commun. 2020 Nov 30;8(1):207.
Robinson T, Abdelhak A, Bose T, Meinl E, Otto M, Zettl UK, Dersch R, Tumani H, Rauer S, Huss A. Cerebrospinal Fluid Biomarkers in Relation to MRZ Reaction Status in Primary Progressive Multiple Sclerosis. Cells. 2020 Nov 25;9(12):2543.
Liu J, Mori M, Zimmermann H, Brandt A, Havla J, Tanaka S, Sugimoto K, Oji S, Uzawa A, Asseyer S, Cooper G, Jarius S, Bellmann-Strobl J, Ruprecht K, Siebert N, Masuda H, Uchida T, Ohtani R, Nomura K, Meinl E, Kuempfel T, Paul F, Kuwabara S. Anti-MOG antibody-associated disorders: differences in clinical profiles and prognosis in Japan and Germany. J Neurol Neurosurg Psychiatry. 2020 Nov 20:jnnp-2020-324422.
Völk S, Unterrainer M, Albert NL, Havla J, Gerdes LA, Schumacher M, Brendel M, Kaiser L, Adorjan K, Rupprecht R, Bartenstein P, Kümpfel T, Danek A. TSPO PET With 18F-GE-180 to Differentiate Variants of Multiple Sclerosis: Relapsing-Remitting Multiple Sclerosis, Tumefactive Demyelination, and Baló's Concentric Sclerosis. Clin Nucl Med. 2020 Oct;45(10):e447-e448.
Grigorescu C, Chalah MA, Lefaucheur JP, Kümpfel T, Padberg F, Ayache SS, Palm U. Effects of Transcranial Direct Current Stimulation on Information Processing Speed, Working Memory, Attention, and Social Cognition in Multiple Sclerosis. Front Neurol. 2020 Oct 15;11:545377.
Khatri S, Psaraftis N, Funaro A, Arinuma Y, Fujieda Y, Mader S, Jørgensen CD, Astakhova K. Serological comparison of systemic lupus erythematosus with neuropsychiatric lupus using synthetic nucleic acid antigens. J Transl Autoimmun. 2020 Oct 28;3:100068.
Specovius S, Zimmermann HG, Oertel FC, Chien C, Bereuter C, Cook LJ, Lana Peixoto MA, Fontenelle MA, Kim HJ, Hyun JW, Jung SK, Palace J, Roca-Fernandez A, Diaz AR, Leite MI, Sharma SM, Ashtari F, Kafieh R, Dehghani A, Pourazizi M, Pandit L, Dcunha A, Aktas O, Ringelstein M, Albrecht P, May E, Tongco C, Leocani L, Pisa M, Radaelli M, Martinez-Lapiscina EH, Stiebel-Kalish H, Hellmann M, Lotan I, Siritho S, de Seze J, Senger T, Havla J, Marignier R, Tilikete C, Cobo Calvo A, Bichuetti DB, Tavares IM, Asgari N, Soelberg K, Altintas A, Yildirim R, Tanriverdi U, Jacob A, Huda S, Rimler Z, Reid A, Mao-Draayer Y, de Castillo IS, Yeaman MR, Smith TJ, Brandt AU, Paul F; GJCF International Clinical Consortium for NMOSD. Cohort profile: a collaborative multicentre study of retinal optical coherence tomography in 539 patients with neuromyelitis optica spectrum disorders (CROCTINO). BMJ Open. 2020 Oct 29;10(10):e035397.
Snaidero N, Schifferer M, Mezydlo A, Zalc B, Kerschensteiner M*, Misgeld T. Myelin replacement triggered by single-cell demyelination in mouse cortex. Nat Commun. 2020 Sep 29;11(1):4901.
Matthias J, Heink S, Picard F, Zeiträg J, Kolz A, Chao YY, Soll D, de Almeida GP, Glasmacher E, Jacobsen ID, Riedel T, Peters A, Floess S, Huehn J, Baumjohann D, Huber M, Korn T, Zielinski CE. Salt generates antiinflammatory Th17 cells but amplifies pathogenicity in proinflammatory cytokine microenvironments. J Clin Invest. 2020 Sep 1;130(9):4587-4600.
Saxena S, Liebscher S: Editorial: Circuit Mechanisms of Neurodegenerative Diseases. Front Neurosci 2020, 14:593329.
Jarius S, Pellkofer H, Siebert N, Korporal-Kuhnke M, Hümmert MW, Ringelstein M, Rommer PS, Ayzenberg I, Ruprecht K, Klotz L, Asgari N, Zrzavy T, Höftberger R, Tobia R, Buttmann M, Fechner K, Schanda K, Weber M, Asseyer S, Haas J, Lechner C, Kleiter I, Aktas O, Trebst C, Rostasy K, Reindl M, Kümpfel T, Paul F, Wildemann B; in cooperation with the Neuromyelitis Optica Study Group (NEMOS). Cerebrospinal fluid findings in patients with myelin oligodendrocyte glycoprotein (MOG) antibodies. Part 1: Results from 163 lumbar punctures in 100 adult patients. J Neuroinflammation. 2020 Sep 3;17(1):261.
Gerdes LA, Janoschka C, Eveslage M, Mannig B, Wirth T, Schulte-Mecklenbeck A, Lauks S, Glau L, Gross CC, Tolosa E, Flierl-Hecht A, Ertl-Wagner B, Barkhof F, Meuth SG, Kümpfel T, Wiendl H, Hohlfeld R, Klotz L. Immune signatures of prodromal multiple sclerosis in monozygotic twins. Proc Natl Acad Sci U S A. 2020 Sep 1;117(35):21546-21556.
Lichtenthaler SF, Meinl E. To cut or not to cut: New rules for proteolytic shedding of membrane proteins. J Biol Chem. 2020 Aug 28;295(35):12353-12354.
Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, Cross AH, de Seze J, Leppert D, Montalban X, Selmaj K, Wiendl H, Kerloeguen C, Willi R, Li B, Kakarieka A, Tomic D, Goodyear A, Pingili R, Häring DA, Ramanathan K, Merschhemke M, Kappos L; ASCLEPIOS I and ASCLEPIOS II Trial Groups. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N Engl J Med. 2020 Aug 6;383(6):546-557.
Havla J, Moser M, Sztatecsny C, Lotz-Havla AS, Maier EM, Hizli B, Schinner R, Kümpfel T, Strupp M, Bremova-Ertl T, Schneider SA. Retinal axonal degeneration in Niemann-Pick type C disease. J Neurol. 2020 Jul;267(7):2070-2082.
Inan B, Gocmen R, Vural A, Colpak AI, Meinl E, Karabudak R, Tuncer A. Myelin oligodendrocyte glycoprotein antibody associated central nervous system demyelinating disease: a tertiary center experience from Turkey. Mult Scler Relat Disord. 2020 Jul 4;44:102376.
Kislinger G, Gnägi H, Kerschensteiner M, Simons M, Misgeld T, Schifferer M. Multiscale ATUM-FIB Microscopy Enables Targeted Ultrastructural Analysis at Isotropic Resolution. iScience. 2020 Jul 24;23(7):101290.
Sauerbeck AD, Gangolli M, Reitz SJ, Salyards MH, Kim SH, Hemingway C, Gratuze M, Makkapati T, Kerschensteiner M, Holtzman DM, Brody DL, Kummer TT. SEQUIN Multiscale Imaging of Mammalian Central Synapses Reveals Loss of Synaptic Connectivity Resulting from Diffuse Traumatic Brain Injury. Neuron. 2020 Jul 22;107(2):257-273.e5.
Gasperi C, Andlauer TFM, Keating A, Knier B, Klein A, Pernpeintner V, Lichtner P, Gold R, Zipp F, Then Bergh F, Stangel M, Tumani H, Wildemann B, Wiendl H, Bayas A, Kümpfel T, Zettl UK, Linker RA, Ziemann U, Knop M, Warnke C, Friese MA, Paul F, Tackenberg B, Berthele A, Hemmer B. Genetic determinants of the humoral immune response in MS. Neurol Neuroimmunol Neuroinflamm. 2020 Jul 16;7(5):e827.
Gunes ZI, Kan VWY, Ye X, Liebscher S: Exciting Complexity: The Role of Motor Circuit Elements in ALS Pathophysiology. Front Neurosci 2020, 14:573.
Aly L, Havla J, Lepennetier G, Andlauer TFM, Sie C, Strauß EM, Hoshi MM, Kümpfel T, Hiltensperger M, Mitsdoerffer M, Mühlau M, Zimmer C, Hemmer B, Korn T, Knier B. Inner retinal layer thinning in radiologically isolated syndrome predicts conversion to multiple sclerosis. Eur J Neurol. 2020 Jun 26. doi: 10.1111/ene.14416. Epub ahead of print.
Mulazzani E, Wagner D, Havla J, Schlüter M, Meinl I, Gerdes LA, Kümpfel T. Neurological phenotypes in patients with NLRP3-, MEFV-, and TNFRSF1A low-penetrance variants. J Neuroinflammation. 2020 Jun 20;17(1):196.
Thaler FS, Bangol B, Biljecki M, Havla J, Schumacher AM, Kümpfel T. Possible link of genetic variants to autoimmunity in GAD-antibody-associated neurological disorders. J Neurol Sci. 2020 Jun 15;413:116860.
Mader S, Kümpfel T, Meinl E. Novel insights into pathophysiology and therapeutic possibilities reveal further differences between AQP4-IgG- and MOG-IgG-associated diseases. Curr Opin Neurol. 2020 Jun;33(3):362-371.
D'Souza M, Papadopoulou A, Levy M, Jacob A, Yeaman MR, Kümpfel T, Marignier R, Paul F, Brandt AU. Diagnostic procedures in suspected attacks in patients with neuromyelitis optica spectrum disorders: Results of an international survey. Mult Scler Relat Disord. 2020 Jun;41:102027.
Chalah MA, Grigorescu C, Padberg F, Kümpfel T, Palm U, Ayache SS. Bifrontal transcranial direct current stimulation modulates fatigue in multiple sclerosis: a randomized sham-controlled study. J Neural Transm. 2020 Jun;127(6):953-961.
Motamedi S, Oertel FC, Yadav SK, Kadas EM, Weise M, Havla J, Ringelstein M, Aktas O, Albrecht P, Ruprecht K, Bellmann-Strobl J, Zimmermann HG, Paul F, Brandt AU. Altered fovea in AQP4-IgG-seropositive neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2020 Jun 23;7(5):e805.
Tenembaum S, Yeh EA; Guthy-Jackson Foundation International Clinical Consortium (GJCF-ICC). Pediatric NMOSD: A Review and Position Statement on Approach to Work-Up and Diagnosis. Front Pediatr. 2020 Jun 25;8:339.
The iMSMS Consortium. Household paired design reduces variance and increases power in multi-city gut microbiome study in multiple sclerosis. Mult Scler. 2020 Jun 26:1352458520924594.
Abdelhak A, Huss A, Stahmann A, Senel M, Krumbholz M, Kowarik MC, Havla J, Kümpfel T, Kleiter I, Wüstinger I, Zettl UK, Schwartz M, Roesler R, Friede T, Ludolph AC, Ziemann U, Tumani H. Explorative study of emerging blood biomarkers in progressive multiple sclerosis (EmBioProMS): Design of a prospective observational multicentre pilot study. Contemp Clin Trials Commun. 2020 May 19;18:100574.
Abrahamyan S, Eberspächer B, Hoshi MM, Aly L, Luessi F, Groppa S, Klotz L, Meuth SG, Schroeder C, Grüter T, Tackenberg B, Paul F, Then-Bergh F, Kümpfel T, Weber F, Stangel M, Bayas A, Wildemann B, Heesen C, Zettl U, Warnke C, Antony G, Hessler N, Wiendl H, Bittner S, Hemmer B, Gold R, Salmen A, Ruprecht K; German Competence Network Multiple Sclerosis (KKNMS); Other members of the KKNMS that acted as collaborators in this study. Complete Epstein-Barr virus seropositivity in a large cohort of patients with early multiple sclerosis. J Neurol Neurosurg Psychiatry. 2020 Jul;91(7):681-686.
Granier C, Schwarting J, Fourli E, Laage-Gaupp F, Hennrich AA, Schmalz A, Jacobi A, Wesolowski M, Conzelmann KK, Bareyre FM. Formation of somatosensory detour circuits mediates functional recovery following dorsal column injury. Sci Rep. 2020 Jul 2;10(1):10953.
Kleiter I, Ayzenberg I, Havla J, Lukas C, Penner IK, Stadelmann C, Linker RA. The transitional phase of multiple sclerosis: Characterization and conceptual framework. Mult Scler Relat Disord. 2020 May 29;44:102242. doi: 10.1016/j.msard.2020.102242.
Engel S, Graetz C, Salmen A, Muthuraman M, Toenges G, Ambrosius B, Bayas A, Berthele A, Heesen C, Klotz L, Kümpfel T, Linker RA, Meuth SG, Paul F, Stangel M, Tackenberg B, Then Bergh F, Tumani H, Weber F, Wildemann B, Zettl UK, Antony G, Bittner S, Groppa S, Hemmer B, Wiendl H, Gold R, Zipp F, Lill CM, Luessi F; German Competence Network of Multiple Sclerosis. Is APOE ε4 associated with cognitive performance in early MS? Neurol Neuroimmunol Neuroinflamm. 2020 May 1;7(4):e728.
Heitmann H, Haller B, Tiemann L, Mühlau M, Berthele A, Tölle TR, Salmen A, Ambrosius B, Bayas A, Asseyer S, Hartung HP, Heesen C, Stangel M, Wildemann B, Haars S, Groppa S, Luessi F, Kümpfel T, Nischwitz S, Meuth SG, Klotz L, Linker RA, Zettl UK, Ziemann U, Tumani H, Tackenberg B, Zipp F, Wiendl H, Gold R, Hemmer B, Ploner M. Longitudinal prevalence and determinants of pain in multiple sclerosis: results from the German National Multiple Sclerosis Cohort study. Pain. 2020 Apr;161(4):787-796.
Dietrich M, Koska V, Hecker C, Göttle P, Hilla AM, Heskamp A, Lepka K, Issberner A, Hallenberger A, Baksmeier C, Steckel J, Balk L, Knier B, Korn T, Havla J, Martínez-Lapiscina EH, Solà-Valls N, Manogaran P, Olbert ED, Schippling S, Cruz-Herranz A, Yiu H, Button J, Caldito NG, von Gall C, Mausberg AK, Stettner M, Zimmermann HG, Paul F, Brandt AU, Küry P, Goebels N, Aktas O, Berndt C, Saidha S, Green AJ, Calabresi PA, Fischer D, Hartung HP, Albrecht P. Protective effects of 4-aminopyridine in experimental optic neuritis and multiple sclerosis. Brain. 2020 Apr 1;143(4):1127-1142.
Teuber-Hanselmann S, Meinl E, Junker A. MicroRNAs in gray and white matter multiple sclerosis lesions: impact on pathophysiology. J Pathol. 2020 Apr;250(5):496-509.
Huber JE, Chang Y, Meinl I, Kümpfel T, Meinl E, Baumjohann D. Fingolimod Profoundly Reduces Frequencies and Alters Subset Composition of Circulating T Follicular Helper Cells in Multiple Sclerosis Patients. J Immunol. 2020 Mar 1;204(5):1101-1110.
Selge C, Kümpfel T, Havla J, Schöberl F, Danek A, Reilich P. Seronegatives myasthenes Syndrom? [Seronegative myasthenic syndrome?]. Nervenarzt. 2020 Feb;91(2):150-152.
Stork L, Ellenberger D, Ruprecht K, Reindl M, Beißbarth T, Friede T, Kümpfel T, Gerdes LA, Gloth M, Liman T, Paul F, Brück W, Metz I. Antibody signatures in patients with histopathologically defined multiple sclerosis patterns. Acta Neuropathol. 2020 Mar;139(3):547-564.
Faissner S, Graz F, Reinehr S, Petrikowski L, Haupeltshofer S, Ceylan U, Stute G, Winklmeier S, Pache F, Paul F, Ruprecht K, Meinl E, Dick HB, Gold R, Kleiter I, Joachim SC. Binding patterns and functional properties of human antibodies to AQP4 and MOG on murine optic nerve and retina. J Neuroimmunol. 2020 Feb 20;342:577194.
Bracher A, Alcalá C, Ferrer J, Melzer N, Hohlfeld R, Casanova B, Beltrán E, Dornmair K. An expanded parenchymal CD8+ T cell clone in GABAA receptor encephalitis. Ann Clin Transl Neurol. 2020 Feb;7(2):239-244.
Gómez-Fernández P, Lopez de Lapuente Portilla A, Astobiza I, Mena J, Urtasun A, Altmann V, Matesanz F, Otaegui D, Urcelay E, Antigüedad A, Malhotra S, Montalban X, Castillo-Triviño T, Espino-Paisán L, Aktas O, Buttmann M, Chan A, Fontaine B, Gourraud PA, Hecker M, Hoffjan S, Kubisch C, Kümpfel T, Luessi F, Zettl UK, Zipp F, Alloza I, Comabella M, Lill CM, Vandenbroeck K. The Rare IL22RA2 Signal Peptide Coding Variant rs28385692 Decreases Secretion of IL-22BP Isoform-1, -2 and -3 and Is Associated with Risk for Multiple Sclerosis. Cells. 2020 Jan 10;9(1):175.
Ringelstein M, Harmel J, Zimmermann H, Brandt AU, Paul F, Haarmann A, Buttmann M, Hümmert MW, Trebst C, Schroeder C, Ayzenberg I, Kleiter I, Hellwig K, Havla J, Kümpfel T, Jarius S, Wildemann B, Rommer P, Weber MS, Pellkofer H, Röpke L, Geis C, Retzlaff N, Zettl U, Deppe M, Klotz L, Young K, Stellmann JP, Kaste M, Kermer P, Marouf W, Lauda F, Tumani H, Graf J, Klistorner A, Hartung HP, Aktas O, Albrecht P; Neuromyelitis Optica Study Group (NEMOS). Longitudinal optic neuritis-unrelated visual evoked potential changes in NMO spectrum disorders. Neurology. 2020 Jan 28;94(4):e407-e418.
2019
Schumacher AM, Misgeld T, Kerschensteiner M, Snaidero N. Imaging the execution phase of neuroinflammatory disease models. Exp Neurol. 2019 Oct;320:112968.
Schönecker S, Brendel M, Palleis C, Beyer L, Höglinger GU, Schuh E, Rauchmann BS, Sauerbeck J, Rohrer G, Sonnenfeld S, Furukawa K, Ishiki A, Okamura N, Bartenstein P, Dieterich M, Bötzel K, Danek A, Rominger A, Levin J. PET Imaging of Astrogliosis and Tau Facilitates Diagnosis of Parkinsonian Syndromes. Front Aging Neurosci. 2019 Sep 11;11:249.
Schönecker S, Brendel M, Palleis C, Beyer L, Höglinger GU, Schuh E, Rauchmann BS, Sauerbeck J, Rohrer G, Sonnenfeld S, Furukawa K, Ishiki A, Okamura N, Bartenstein P, Dieterich M, Bötzel K, Danek A, Rominger A, Levin J. PET Imaging of Astrogliosis and Tau Facilitates Diagnosis of Parkinsonian Syndromes. Front Aging Neurosci. 2019 Sep 11;11:249.
Denecke CK, Aljović A, Bareyre FM. Combining molecular intervention with in vivo imaging to untangle mechanisms of axon pathology and outgrowth following spinal cord injury. Exp Neurol. 2019 Aug;318:1-11.
Konjevic Sabolek M, Held K, Beltrán E, Niedl AG, Meinl E, Hohlfeld R, Lassmann H, Dornmair K. Communication of CD8+ T cells with mononuclear phagocytes in multiple sclerosis. Ann Clin Transl Neurol. 2019 Jul;6(7):1151-1164.
Gunia-Krzyżak A, Bareyre FM, Marona H, Waszkielewicz AM. Four N-(E)-cinnamoyl (cinnamamide) derivatives of aminoalkanols with promising anticonvulsant and analgesic activity. Bioorg Med Chem Lett. 2019 Jun 1;29(11):1298-1303.
Marti Fernandez I, Macrini C, Krumbholz M, Hensbergen PJ, Hipgrave Ederveen AL, Winklmeier S, Vural A, Kurne A, Jenne D, Kamp F, Gerdes LA, Hohlfeld R, Wuhrer M, Kümpfel T, Meinl E. The Glycosylation Site of Myelin Oligodendrocyte Glycoprotein Affects Autoantibody Recognition in a Large Proportion of Patients. Front Immunol. 2019 Jun 11;10:1189.
International Multiple Sclerosis Genetics Consortium. International Multiple Sclerosis Genetics Consortium. Low-Frequency and Rare-Coding Variation Contributes to Multiple Sclerosis Risk. Cell. 2019 Jun 27;178(1):262.
Schuh E, Groß CJ, Wagner D, Schlüter M, Groß O, Kümpfel T. MCC950 blocks enhanced interleukin-1β production in patients with NLRP3 low penetrance variants. Clin Immunol. 2019 Jun;203:45-52.
Nolan-Kenney RC, Liu M, Akhand O, Calabresi PA, Paul F, Petzold A, Balk L, Brandt AU, Martínez-Lapiscina EH, Saidha S, Villoslada P, Al-Hassan AA, Behbehani R, Frohman EM, Frohman T, Havla J, Hemmer B, Jiang H, Knier B, Korn T, Leocani L, Papadopoulou A, Pisa M, Zimmermann H, Galetta SL, Balcer LJ; International Multiple Sclerosis Visual System Consortium. Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: An international study. Ann Neurol. 2019 May;85(5):618-629.
Förster M, Küry P, Aktas O, Warnke C, Havla J, Hohlfeld R, Mares J, Hartung HP, Kremer D. Managing Risks with Immune Therapies in Multiple Sclerosis. Drug Saf. 2019 May;42(5):633-647.
Thaler FS, Thaller AL, Biljecki M, Schuh E, Winklmeier S, Mahler CF, Gerhards R, Völk S, Schnorfeil F, Subklewe M, Hohlfeld R, Kümpfel T, Meinl E. Abundant glutamic acid decarboxylase (GAD)-reactive B cells in gad-antibody-associated neurological disorders. Ann Neurol. 2019 Mar;85(3):448-454.
Hohlfeld R. Immune dysbalance in childhood multiple sclerosis: a 'chicken or the egg' conundrum. Brain. 2019 Mar 1;142(3):490-492.
Mader S, Brimberg L. Aquaporin-4 Water Channel in the Brain and Its Implication for Health and Disease. Cells. 2019 Jan 27;8(2):90.
Harnisch K, Teuber-Hanselmann S, Macha N, Mairinger F, Fritsche L, Soub D, Meinl E, Junker A. Myelination in Multiple Sclerosis Lesions Is Associated with Regulation of Bone Morphogenetic Protein 4 and Its Antagonist Noggin. Int J Mol Sci. 2019 Jan 3;20(1):154.
Bronge M, Ruhrmann S, Carvalho-Queiroz C, Nilsson OB, Kaiser A, Holmgren E, Macrini C, Winklmeier S, Meinl E, Brundin L, Khademi M, Olsson T, Gafvelin G, Grönlund H. Myelin oligodendrocyte glycoprotein revisited-sensitive detection of MOG-specific T-cells in multiple sclerosis. J Autoimmun. 2019 Aug;102:38-49.
Beltrán E, Nguyen XH, Quériault C, Barateau L, Dauvilliers Y, Dornmair K, Liblau RS. Shared T cell receptor chains in blood memory CD4+ T cells of narcolepsy type 1 patients. J Autoimmun. 2019 Jun;100:1-6.
Beltrán E, Gerdes LA, Hansen J, Flierl-Hecht A, Krebs S, Blum H, Ertl-Wagner B, Barkhof F, Kümpfel T, Hohlfeld R, Dornmair K. Early adaptive immune activation detected in monozygotic twins with prodromal multiple sclerosis. J Clin Invest. 2019 Nov 1;129(11):4758-4768.
Bradley PM, Denecke CK, Aljovic A, Schmalz A, Kerschensteiner M, Bareyre FM. Corticospinal circuit remodeling after central nervous system injury is dependent on neuronal activity. J Exp Med. 2019 Nov 4;216(11):2503-2514.
Mulazzani M, Huber M, Borchard S, Langer S, Angele B, Schuh E, Meinl E, Dreyling M, Birnbaum T, Straube A, Koedel U, von Baumgarten L. APRIL and BAFF: novel biomarkers for central nervous system lymphoma. J Hematol Oncol. 2019 Oct 15;12(1):102.
Traub JW, Pellkofer HL, Grondey K, Seeger I, Rowold C, Brück W, Husseini L, Häusser-Kinzel S, Weber MS. Natalizumab promotes activation and pro-inflammatory differentiation of peripheral B cells in multiple sclerosis patients. J Neuroinflammation. 2019 Nov 16;16(1):228.
Oertel FC, Outteryck O, Knier B, Zimmermann H, Borisow N, Bellmann-Strobl J, Blaschek A, Jarius S, Reindl M, Ruprecht K, Meinl E, Hohlfeld R, Paul F, Brandt AU, Kümpfel T, Havla J. Optical coherence tomography in myelin-oligodendrocyte-glycoprotein antibody-seropositive patients: a longitudinal study. J Neuroinflammation. 2019 Jul 25;16(1):154.
Johnen A, Bürkner PC, Landmeyer NC, Ambrosius B, Calabrese P, Motte J, Hessler N, Antony G, König IR, Klotz L, Hoshi MM, Aly L, Groppa S, Luessi F, Paul F, Tackenberg B, Bergh FT, Kümpfel T, Tumani H, Stangel M, Weber F, Bayas A, Wildemann B, Heesen C, Zettl UK, Zipp F, Hemmer B, Meuth SG, Gold R, Wiendl H, Salmen A; German Competence Network Multiple Sclerosis (KKNMS). Can we predict cognitive decline after initial diagnosis of multiple sclerosis? Results from the German National early MS cohort (KKNMS). J Neurol. 2019 Feb;266(2):386-397.
Gasperi C, Salmen A, Antony G, Bayas A, Heesen C, Kümpfel T, Linker RA, Paul F, Stangel M, Tackenberg B, Bergh FT, Warnke C, Weber F, Wiendl H, Wildemann B, Zettl UK, Ziemann U, Zipp F, Tumani H, Gold R, Hemmer B; German Competence Network of Multiple Sclerosis. Association of Intrathecal Immunoglobulin G Synthesis With Disability Worsening in Multiple Sclerosis. JAMA Neurol. 2019 Jul 1;76(7):841-849.
Hohlfeld R. Immune checkpoint blockade for treating progressive multifocal leukoencephalopathy. Lancet Neurol. 2019 Jul;18(7):623-624.
Graetz C, Gröger A, Luessi F, Salmen A, Zöller D, Schultz J, Siller N, Fleischer V, Bellenberg B, Berthele A, Biberacher V, Havla J, Hecker M, Hohlfeld R, Infante-Duarte C, Kirschke JS, Kümpfel T, Linker R, Paul F, Pfeuffer S, Sämann P, Toenges G, Weber F, Zettl UK, Jahn-Eimermacher A, Antony G, Groppa S, Wiendl H, Hemmer B, Mühlau M, Lukas C, Gold R, Lill CM, Zipp F. Association of smoking but not HLA-DRB1*15:01, APOE or body mass index with brain atrophy in early multiple sclerosis. Mult Scler. 2019 Apr;25(5):661-668.
Balk LJ, Coric D, Knier B, Zimmermann HG, Behbehani R, Alroughani R, Martinez-Lapiscina EH, Brandt AU, Sánchez-Dalmau B, Vidal-Jordana A, Albrecht P, Koska V, Havla J, Pisa M, Nolan RC, Leocani L, Paul F, Aktas O, Montalban X, Balcer LJ, Villoslada P, Outteryck O, Korn T, Petzold A; IMSVISUAL consortium. Retinal inner nuclear layer volume reflects inflammatory disease activity in multiple sclerosis; a longitudinal OCT study. Mult Scler J Exp Transl Clin. 2019 Sep 5;5(3):2055217319871582.
Drulovic J, Martinovic V, Basuroski ID, Mesaros S, Mader S, Weinshenker B, Pekmezovic T. Long-term outcome and prognosis in patients with neuromyelitis optica spectrum disorder from Serbia. Mult Scler Relat Disord. 2019 Nov;36:101413.
Eisele P, Konstandin S, Szabo K, Ebert A, Roßmanith C, Paschke N, Kerschensteiner M, Platten M, Schoenberg SO, Schad LR, Gass A. Temporal evolution of acute multiple sclerosis lesions on serial sodium (23Na) MRI. Mult Scler Relat Disord. 2019 Apr;29:48-54
Gross CC, Schulte-Mecklenbeck A, Rünzi A, Kuhlmann T, Posevitz-Fejfár A, Schwab N, Schneider-Hohendorf T, Herich S, Held K, Konjević M, Hartwig M, Dornmair K, Hohlfeld R, Ziemssen T, Klotz L, Meuth SG, Wiendl H. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc Natl Acad Sci U S A. 2016 May 24;113(21):E2973-82.
Souren NY, Gerdes LA, Lutsik P, Gasparoni G, Beltrán E, Salhab A, Kümpfel T, Weichenhan D, Plass C, Hohlfeld R, Walter J. DNA methylation signatures of monozygotic twins clinically discordant for multiple sclerosis. Nat Commun. 2019 May 7;10(1):2094.
Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Forstera B, Zhao S, Bhatia HS, Parra-Damas A, Mrowka L, Theodorou D, et al: Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat Neurosci 2019, 22:317-327.
Bittner S, Engel S, Lange C, Weber MS, Haghikia A, Luessi F, Korn T, Klotz L, Bayas A, Paul F, Heesen C, Stangel M, Wildemann B, Bergh FT, Tackenberg B, Trebst C, Warnke C, Linker R, Kerschensteiner M, Zettl U, Tumani H, Brück W, Meuth SG, Kümpfel T, Hemmer B, Wiendl H, Gold R, Zipp F. Diagnostik und Therapie von Tuberkulose unter Immuntherapien für Multiple Sklerose : Aktueller Stand und Empfehlungen in Deutschland [Diagnostics and treatment of tuberculosis under immunotherapy for multiple sclerosis : Current status and recommendations in Germany]. Nervenarzt. 2019 Dec;90(12):1245-125
Loy K, Bareyre FM. Rehabilitation following spinal cord injury: how animal models can help our understanding of exercise-induced neuroplasticity. Neural Regen Res. 2019 Mar;14(3):405-412.
Stengel H, Vural A, Brunder AM, Heinius A, Appeltshauser L, Fiebig B, Giese F, Dresel C, Papagianni A, Birklein F, Weis J, Huchtemann T, Schmidt C, Körtvelyessy P, Villmann C, Meinl E, Sommer C, Leypoldt F, Doppler K. Anti-pan-neurofascin IgG3 as a marker of fulminant autoimmune neuropathy. Neurol Neuroimmunol Neuroinflamm. 2019 Aug 16;6(5):e603.
Winklmeier S, Schlüter M, Spadaro M, Thaler FS, Vural A, Gerhards R, Macrini C, Mader S, Kurne A, Inan B, Karabudak R, Özbay FG, Esendagli G, Hohlfeld R, Kümpfel T, Meinl E. Identification of circulating MOG-specific B cells in patients with MOG antibodies. Neurol Neuroimmunol Neuroinflamm. 2019 Oct 14;6(6):625. Erratum in: Neurol Neuroimmunol Neuroinflamm. 2019 Nov 15;7(1).
Schubert J, Brämer D, Huttner HB, Gerner ST, Fuhrer H, Melzer N, Dik A, Prüss H, Ly LT, Fuchs K, Leypoldt F, Nissen G, Schirotzek I, Dohmen C, Bösel J, Lewerenz J, Thaler F, Kraft A, Juranek A, Ringelstein M, Sühs KW, Urbanek C, Scherag A, Geis C, Witte OW, Günther A; GENERATE and IGNITE network. Management and prognostic markers in patients with autoimmune encephalitis requiring ICU treatment. Neurol Neuroimmunol Neuroinflamm. 2018 Oct 30;6(1):e514.
Thaler FS, Koriath C, Vollmar C, Bardins S, Kremmyda O, Danek A. Acute frontal eye field infarction: A topodiagnostic challenge. Neurology. 2019 Jan 22;92(4):193-195.
Thaler FS, Koriath C, Vollmar C, Bardins S, Kremmyda O, Danek A. Acute frontal eye field infarction: A topodiagnostic challenge. Neurology. 2019 Jan 22;92(4):193-195.
Witte ME, Schumacher AM, Mahler CF, Bewersdorf JP, Lehmitz J, Scheiter A, Sánchez P, Williams PR, Griesbeck O, Naumann R, Misgeld T, Kerschensteiner M. Calcium Influx through Plasma-Membrane Nanoruptures Drives Axon Degeneration in a Model of Multiple Sclerosis. Neuron. 2019 Feb 20;101(4):615-624
Penter L, Dietze K, Ritter J, Lammoglia Cobo MF, Garmshausen J, Aigner F, Bullinger L, Hackstein H, Wienzek-Lischka S, Blankenstein T, Hummel M, Dornmair K, Hansmann L. Localization-associated immune phenotypes of clonally expanded tumor-infiltrating T cells and distribution of their target antigens in rectal cancer. Oncoimmunology. 2019 Mar 24;8(6):e1586409.
Peters A, Wekerle H. Autoimmune diabetes mellitus and the leaky gut. Proc Natl Acad Sci U S A. 2019 Jul 23;116(30):14788-14790.
Burgold J, Schulz-Trieglaff EK, Voelkl K, Gutiérrez-Ángel S, Bader JM, Hosp F, Mann M, Arzberger T, Klein R, Liebscher S*, Dudanova I: Cortical circuit alterations precede motor impairments in Huntington’s disease mice. Scientific Reports 2019, 9:6634. (* co-senior author)
Jordão MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Tai YH, Tay TL, Schramm E, Armbruster S, Hagemeyer N, Groß O, Mai D, Çiçek Ö, Falk T, Kerschensteiner M, Grün D, Prinz M. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019 Jan 25;363(6425):eaat7554.
Klotz L, Havla J, Schwab N, Hohlfeld R, Barnett M, Reddel S, Wiendl H. Risks and risk management in modern multiple sclerosis immunotherapeutic treatment. Ther Adv Neurol Disord. 2019 Apr 1;12:1756286419836571.
2018
Mueller SH, Färber A, Prüss H, Melzer N, Golombeck KS, Kümpfel T, Thaler F, Elisak M, Lewerenz J, Kaufmann M, Sühs KW, Ringelstein M, Kellinghaus C, Bien CG, Kraft A, Zettl UK, Ehrlich S, Handreka R, Rostásy K, Then Bergh F, Faiss JH, Lieb W, Franke A, Kuhlenbäumer G, Wandinger KP, Leypoldt F; German Network for Research on Autoimmune Encephalitis (GENERATE). Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Ann Neurol. 2018 Apr;83(4):863-869.
Spadaro M, Winklmeier S, Beltrán E, Macrini C, Höftberger R, Schuh E, Thaler FS, Gerdes LA, Laurent S, Gerhards R, Brändle S, Dornmair K, Breithaupt C, Krumbholz M, Moser M, Krishnamoorthy G, Kamp F, Jenne D, Hohlfeld R, Kümpfel T, Lassmann H, Kawakami N, Meinl E. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann Neurol. 2018 Aug;84(2):315-328.
Oviedo-Salcedo T, de Witte L, Kümpfel T, Kahn RS, Falkai P, Eichhorn P, Luykx J, Hasan A. Absence of cerebrospinal fluid antineuronal antibodies in schizophrenia spectrum disorders. Br J Psychiatry. 2018 May;212(5):318-320.
Barrantes-Freer A, Engel AS, Rodríguez-Villagra OA, Winkler A, Bergmann M, Mawrin C, Kuempfel T, Pellkofer H, Metz I, Bleckmann A, Hernández-Durán S, Schippling S, Rushing EJ, Frank S, Glatzel M, Matschke J, Hartmann C, Reifenberger G, Müller W, Schildhaus HU, Brück W, Stadelmann C. Diagnostic red flags: steroid-treated malignant CNS lymphoma mimicking autoimmune inflammatory demyelination. Brain Pathol. 2018 Mar;28(2):225-233.
International Multiple Sclerosis Genetics Consortium. Low-Frequency and Rare-Coding Variation Contributes to Multiple Sclerosis Risk. Cell. 2020 Jan 23;180(2):403.
Hohlfeld R. B-cells as therapeutic targets in neuro-inflammatory diseases. Clin Immunol. 2018 Jan;186:51-53.
Unterrainer M, Diekmann C, Dorostkar M, Vettermann FJ, Kümpfel T, Tonn JC, Bartenstein P, Albert NL. Neurosarcoidosis Mimics High-Grade Glioma in Dynamic 18F-FET PET Due to LAT Expression. Clin Nucl Med. 2018 Nov;43(11):840-841.
Deussing M, Blume T, Vomacka L, Mahler C, Focke C, Todica A, Unterrainer M, Albert NL, Lindner S, von Ungern-Sternberg B, Baumann K, Zwergal A, Bartenstein P, Herms J, Rominger A, Brendel M. Data on specificity of [18F]GE180 uptake for TSPO expression in rodent brain and myocardium. Data Brief. 2018 May 5;19:331-336.
Unterrainer M, Mahler C, Vomacka L, Lindner S, Havla J, Brendel M, Böning G, Ertl-Wagner B, Kümpfel T, Milenkovic VM, Rupprecht R, Kerschensteiner M, Bartenstein P, Albert NL. TSPO PET with [18F]GE-180 sensitively detects focal neuroinflammation in patients with relapsing-remitting multiple sclerosis. Eur J Nucl Med Mol Imaging. 2018 Jul;45(8):1423-1431.
Blaschek A, V Kries R, Lohse P, Huss K, Vill K, Belohradsky BH, Heinen F, Müller-Felber W, Kümpfel T. TNFRSF1A and MEFV mutations in childhood onset multiple sclerosis. Eur J Paediatr Neurol. 2018 Jan;22(1):72-81.
Vural A, Doppler K, Meinl E. Autoantibodies Against the Node of Ranvier in Seropositive Chronic Inflammatory Demyelinating Polyneuropathy: Diagnostic, Pathogenic, and Therapeutic Relevance. Front Immunol. 2018 May 14;9:1029.
Diederich JM, Staudt M, Meisel C, Hahn K, Meinl E, Meisel A, Klehmet J. Neurofascin and Compact Myelin Antigen-Specific T Cell Response Pattern in Chronic Inflammatory Demyelinating Polyneuropathy Subtypes. Front Neurol. 2018 Mar 19;9:171.
Brendel M, Schönecker S, Höglinger G, Lindner S, Havla J, Blautzik J, Sauerbeck J, Rohrer G, Zach C, Vettermann F, Lang AE, Golbe L, Nübling G, Bartenstein P, Furukawa K, Ishiki A, Bötzel K, Danek A, Okamura N, Levin J, Rominger A. [18F]-THK5351 PET Correlates with Topology and Symptom Severity in Progressive Supranuclear Palsy. Front Aging Neurosci. 2018 Jan 17;9:440.
Sommer NN, Saam T, Coppenrath E, Kooijman H, Kümpfel T, Patzig M, Beyer SE, Sommer WH, Reiser MF, Ertl-Wagner B, Treitl KM. Multiple Sclerosis: Improved Detection of Active Cerebral Lesions With 3-Dimensional T1 Black-Blood Magnetic Resonance Imaging Compared With Conventional 3-Dimensional T1 GRE Imaging. Invest Radiol. 2018 Jan;53(1):13-19.
Arakawa A, Vollmer S, Besgen P, Galinski A, Summer B, Kawakami Y, Wollenberg A, Dornmair K, Spannagl M, Ruzicka T, Thomas P, Prinz JC. Unopposed IL-36 Activity Promotes Clonal CD4+ T-Cell Responses with IL-17A Production in Generalized Pustular Psoriasis. J Invest Dermatol. 2018 Jun;138(6):1338-1347.
Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, Franciotta D, Fujihara K, Jacob A, Kim HJ, Kleiter I, Kümpfel T, Levy M, Palace J, Ruprecht K, Saiz A, Trebst C, Weinshenker BG, Wildemann B. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018 May 3;15(1):134.
Trillsch F, Eichhorn P, Oliveira-Ferrer L, Kuempfel T, Burges A, Mahner S, Havla J. No need for NMDA receptor antibody screening in neurologically asymptomatic patients with ovarian teratomas. J Neurol. 2018 Feb;265(2):431-432.
Doppler K, Stengel H, Appeltshauser L, Grosskreutz J, Man Ng JK, Meinl E, Sommer C. Neurofascin-155 IgM autoantibodies in patients with inflammatory neuropathies. J Neurol Neurosurg Psychiatry. 2018 Nov;89(11):1145-1151.
Oertel FC, Havla J, Roca-Fernández A, Lizak N, Zimmermann H, Motamedi S, Borisow N, White OB, Bellmann-Strobl J, Albrecht P, Ruprecht K, Jarius S, Palace J, Leite MI, Kuempfel T, Paul F, Brandt AU. Retinal ganglion cell loss in neuromyelitis optica: a longitudinal study. J Neurol Neurosurg Psychiatry. 2018 Dec;89(12):1259-1265.
Thaler FS, Catak C, Einhäupl M, Müller S, Seelos K, Wollenweber FA, Kümpfel T. Cerebral small vessel disease caused by a novel heterozygous mutation in HTRA1. J Neurol Sci. 2018 May 15;388:19-21.
Loy K, Schmalz A, Hoche T, Jacobi A, Kreutzfeldt M, Merkler D, Bareyre FM. Enhanced Voluntary Exercise Improves Functional Recovery following Spinal Cord Injury by Impacting the Local Neuroglial Injury Response and Supporting the Rewiring of Supraspinal Circuits. J Neurotrauma. 2018 Dec 15;35(24):2904-2915.
Kapoor R, Ho PR, Campbell N, Chang I, Deykin A, Forrestal F, Lucas N, Yu B, Arnold DL, Freedman MS, Goldman MD, Hartung HP, Havrdová EK, Jeffery D, Miller A, Sellebjerg F, Cadavid D, Mikol D, Steiner D; ASCEND investigators. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018 May;17(5):405-415.
Kawakami N. Intravital Imaging of T Cells Within the Spinal Cord. Methods Mol Biol. 2018;1763:119-127.
Meinl I, Havla J, Hohlfeld R, Kümpfel T. Recurrence of disease activity during pregnancy after cessation of fingolimod in multiple sclerosis. Mult Scler. 2018 Jun;24(7):991-994.
Locatelli G, Theodorou D, Kendirli A, Jordão MJC, Staszewski O, Phulphagar K, Cantuti-Castelvetri L, Dagkalis A, Bessis A, Simons M, Meissner F, Prinz M, Kerschensteiner M. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat Neurosci. 2018 Sep;21(9):1196-1208.
Trebst C, Kümpfel T. Neuroimmunologie und Rheumatologie: Schnittmengen und Differenzialdiagnosen [Neuroimmunology and rheumatology: overlap and differential diagnoses]. Nervenarzt. 2018 Oct;89(10):1095-1105.
Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, Franciotta D, Fujihara K, Jacob A, Kim HJ, Kleiter I, Kümpfel T, Levy M, Palace J, Ruprecht K, Saiz A, Trebst C, Weinshenker BG, Wildemann B. MOG-Enzephalomyelitis: Internationale Empfehlungen zu Diagnose und Antikörpertestung [MOG encephalomyelitis: international recommendations on diagnosis and antibody testing]. Nervenarzt. 2018 Dec;89(12):1388-1399.
Deussing M, Blume T, Vomacka L, Mahler C, Focke C, Todica A, Unterrainer M, Albert NL, Lindner S, von Ungern-Sternberg B, Baumann K, Zwergal A, Bartenstein P, Herms J, Rominger A, Brendel M. Coupling between physiological TSPO expression in brain and myocardium allows stabilization of late-phase cerebral [18F]GE180 PET quantification. Neuroimage. 2018 Jan 15;165:83-91.
Kleiter I, Gahlen A, Borisow N, Fischer K, Wernecke KD, Hellwig K, Pache F, Ruprecht K, Havla J, Kümpfel T, Aktas O, Hartung HP, Ringelstein M, Geis C, Kleinschnitz C, Berthele A, Hemmer B, Angstwurm K, Stellmann JP, Schuster S, Stangel M, Lauda F, Tumani H, Mayer C, Krumbholz M, Zeltner L, Ziemann U, Linker R, Schwab M, Marziniak M, Then Bergh F, Hofstadt-van Oy U, Neuhaus O, Zettl UK, Faiss J, Wildemann B, Paul F, Jarius S, Trebst C; NEMOS (Neuromyelitis Optica Study Group). Apheresis therapies for NMOSD attacks: A retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol Neuroinflamm. 2018 Sep 26;5(6):e504.
von Bismarck O, Dankowski T, Ambrosius B, Hessler N, Antony G, Ziegler A, Hoshi MM, Aly L, Luessi F, Groppa S, Klotz L, Meuth SG, Tackenberg B, Stoppe M, Then Bergh F, Tumani H, Kümpfel T, Stangel M, Heesen C, Wildemann B, Paul F, Bayas A, Warnke C, Weber F, Linker RA, Ziemann U, Zettl UK, Zipp F, Wiendl H, Hemmer B, Gold R, Salmen A. Treatment choices and neuropsychological symptoms of a large cohort of early MS. Neurol Neuroimmunol Neuroinflamm. 2018 Mar 1;5(3):e446.
Mahler C, Unterrainer M, Muth C, Egensperger R, Vomacka L, Lindner S, Ertl-Wagner B, Patzig M, Bartenstein P, Albert N, Kerschensteiner M, Kümpfel T. Imaging microglial activation in tacrolimus-associated CNS vasculitis with translocator protein PET. Neurology. 2018 Nov 13;91(20):936-937.
Schneider-Hohendorf T, Görlich D, Savola P, Kelkka T, Mustjoki S, Gross CC, Owens GC, Klotz L, Dornmair K, Wiendl H, Schwab N. Sex bias in MHC I-associated shaping of the adaptive immune system. Proc Natl Acad Sci U S A. 2018 Feb 27;115(9):2168-2173.
Hoffmann F, Kraft A, Heigl F, Mauch E, Koehler J, Harms L, Kümpfel T, Köhler W, Ehrlich S, Bayas A, Weinmann-Menke J, Beuker C, Henn KH, Ayzenberg I, Ellrichmann G, Hellwig K, Klingel R, Fassbender CM, Fritz H, Slowinski T, Weihprecht H, Brand M, Stiegler T, Galle J, Schimrigk S. Tryptophan immunoadsorption during pregnancy and breastfeeding in patients with acute relapse of multiple sclerosis and neuromyelitis optica. Ther Adv Neurol Disord. 2018 May 28;11:1756286418774973.
Meinl E, Thaler FS, Lichtenthaler SF. Shedding of BAFF/APRIL Receptors Controls B Cells. Trends Immunol. 2018 Sep;39(9):673-676.
2017
Fitzgerald KC, Munger KL, Hartung HP, Freedman MS, Montalbán X, Edan G, Wicklein EM, Radue EW, Kappos L, Pohl C, Ascherio A; BENEFIT Study Group. Sodium intake and multiple sclerosis activity and progression in BENEFIT. Ann Neurol. 2017 Jul;82(1):20-29.
Llovera G, Benakis C, Enzmann G, Cai R, Arzberger T, Ghasemigharagoz A, Mao X, Malik R, Lazarevic I, Liebscher S, Ertürk A, Meissner L, Vivien D, Haffner C, Plesnila N, Montaner J, Engelhardt B, Liesz A. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta Neuropathol. 2017 Dec;134(6):851-868.
Gunia-Krzyżak A, Żesławska E, Bareyre FM, Nitek W, Waszkielewicz AM, Marona H. Physicochemical and biological evaluation of a cinnamamide derivative R,S-(2E)-1-(3-hydroxypiperidin-1-yl)-3-phenylprop-2-en-1-one (KM-608) for nervous system disorders. Chem Biol Drug Des. 2017 Aug;90(2):244-253.
Vomacka L, Albert NL, Lindner S, Unterrainer M, Mahler C, Brendel M, Ermoschkin L, Gosewisch A, Brunegraf A, Buckley C, Kümpfel T, Rupprecht R, Ziegler S, Kerschensteiner M, Bartenstein P, Böning G. TSPO imaging using the novel PET ligand [18F]GE-180: quantification approaches in patients with multiple sclerosis. EJNMMI Res. 2017 Oct 26;7(1):89.
Mazaheri F, Snaidero N, Kleinberger G, Madore C, Daria A, Werner G, Krasemann S, Capell A, Trümbach D, Wurst W, Brunner B, Bultmann S, Tahirovic S, Kerschensteiner M, Misgeld T, Butovsky O, Haass C. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. EMBO Rep. 2017 Jul;18(7):1186-1198.
Kopczak A, Schumacher AM, Nischwitz S, Kümpfel T, Stalla GK, Auer MK. GAD antibody-associated limbic encephalitis in a young woman with APECED. Endocrinol Diabetes Metab Case Rep. 2017 May 25;2017:17-0010.
Ayache SS, Chalah MA, Kümpfel T, Padberg F, Lefaucheur JP, Palm U. Fatigue bei Multipler Sklerose: Neuronale Korrelate und Möglichkeiten nicht-invasiver Hirnstimulation mit tDCS [Multiple sclerosis fatigue, its neural correlates, and its modulation with tDCS]. Fortschr Neurol Psychiatr. 2017 May;85(5):260-269.
Chovsepian A, Empl L, Correa D, Bareyre FM. Heterotopic Transcallosal Projections Are Present throughout the Mouse Cortex. Front Cell Neurosci. 2017 Feb 21;11:36.
Haghayegh Jahromi N, Tardent H, Enzmann G, Deutsch U, Kawakami N, Bittner S, Vestweber D, Zipp F, Stein JV, Engelhardt B. A Novel Cervical Spinal Cord Window Preparation Allows for Two-Photon Imaging of T-Cell Interactions with the Cervical Spinal Cord Microvasculature during Experimental Autoimmune Encephalomyelitis. Front Immunol. 2017 Apr 11;8:406.
Klehmet J, Staudt M, Diederich JM, Siebert E, Meinl E, Harms L, Meisel A. Neurofascin (NF)155- and NF186-Specific T Cell Response in a Patient Developing a Central Pontocerebellar Demyelination after 10 Years of CIDP. Front Neurol. 2017 Dec 22;8:724.
Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, Uhl O, Yao L, Sander LE, Chen CS, Kraus K, de Juan A, Hergenhan SM, Ehlers M, Koletzko B, Haas R, Solbach W, Oster H, Scheiermann C. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity. 2017 Jan 17;46(1):120-132.
Ingrisch M, Sourbron S, Herberich S, Schneider MJ, Kümpfel T, Hohlfeld R, Reiser MF, Ertl-Wagner B. Dynamic Contrast-Enhanced Magnetic Resonance Imaging Suggests Normal Perfusion in Normal-Appearing White Matter in Multiple Sclerosis. Invest Radiol. 2017 Mar;52(3):135-141.
Schuh E, Musumeci A, Thaler FS, Laurent S, Ellwart JW, Hohlfeld R, Krug A, Meinl E. Human Plasmacytoid Dendritic Cells Display and Shed B Cell Maturation Antigen upon TLR Engagement. J Immunol. 2017 Apr 15;198(8):3081-3088.
Hohlfeld R, Steinman L. T Cell-Transfer Experimental Autoimmune Encephalomyelitis: Pillar of Multiple Sclerosis and Autoimmunity. J Immunol. 2017 May 1;198(9):3381-3383.
Hahn S, Trendelenburg G, Scharf M, Denno Y, Brakopp S, Teegen B, Probst C, Wandinger KP, Buttmann M, Haarmann A, Szabados F, Vom Dahl M, Kümpfel T, Eichhorn P, Gold H, Paul F, Jarius S, Melzer N, Stöcker W, Komorowski L. Identification of the flotillin-1/2 heterocomplex as a target of autoantibodies in bona fide multiple sclerosis. J Neuroinflammation. 2017 Jun 23;14(1):123.
Havla J, Kümpfel T, Schinner R, Spadaro M, Schuh E, Meinl E, Hohlfeld R, Outteryck O. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J Neurol. 2017 Jan;264(1):139-151.
Stellmann JP, Krumbholz M, Friede T, Gahlen A, Borisow N, Fischer K, Hellwig K, Pache F, Ruprecht K, Havla J, Kümpfel T, Aktas O, Hartung HP, Ringelstein M, Geis C, Kleinschnitz C, Berthele A, Hemmer B, Angstwurm K, Young KL, Schuster S, Stangel M, Lauda F, Tumani H, Mayer C, Zeltner L, Ziemann U, Linker RA, Schwab M, Marziniak M, Then Bergh F, Hofstadt-van Oy U, Neuhaus O, Zettl U, Faiss J, Wildemann B, Paul F, Jarius S, Trebst C, Kleiter I; NEMOS (Neuromyelitis Optica Study Group). Immunotherapies in neuromyelitis optica spectrum disorder: efficacy and predictors of response. J Neurol Neurosurg Psychiatry. 2017 Aug;88(8):639-647.
Hartl C, Obermeier V, Gerdes LA, Brügel M, von Kries R, Kümpfel T. Seasonal variations of 25-OH vitamin D serum levels are associated with clinical disease activity in multiple sclerosis patients. J Neurol Sci. 2017 Apr 15;375:160-164.
Hohlfeld R, Meinl E. Ocrelizumab in multiple sclerosis: markers and mechanisms. Lancet Neurol. 2017 Apr;16(4):259-261.
Petzold A, Balcer LJ, Calabresi PA, Costello F, Frohman TC, Frohman EM, Martinez-Lapiscina EH, Green AJ, Kardon R, Outteryck O, Paul F, Schippling S, Vermersch P, Villoslada P, Balk LJ; ERN-EYE IMSVISUAL. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017 Oct;16(10):797-812.
Borisow N, Kleiter I, Gahlen A, Fischer K, Wernecke KD, Pache F, Ruprecht K, Havla J, Krumbholz M, Kümpfel T, Aktas O, Ringelstein M, Geis C, Kleinschnitz C, Berthele A, Hemmer B, Angstwurm K, Weissert R, Stellmann JP, Schuster S, Stangel M, Lauda F, Tumani H, Mayer C, Zeltner L, Ziemann U, Linker RA, Schwab M, Marziniak M, Then Bergh F, Hofstadt-van Oy U, Neuhaus O, Winkelmann A, Marouf W, Rückriem L, Faiss J, Wildemann B, Paul F, Jarius S, Trebst C, Hellwig K; NEMOS (Neuromyelitis Optica Study Group). Influence of female sex and fertile age on neuromyelitis optica spectrum disorders. Mult Scler. 2017 Jul;23(8):1092-1103.
Hohlfeld R, Havla J, Kümpfel T. Patient-to-patient transmission of natalizumab-associated PML? Mult Scler. 2017 Oct;23(11):1564-1565.
Hohlfeld R. Stop inflammation and you stop neurodegeneration in MS - Commentary. Mult Scler. 2017 Sep;23(10):1323-1324.
Schöberl F, Csanadi E, Eren O, Dieterich M, Kümpfel T. NMOSD triggered by yellow fever vaccination - An unusual clinical presentation with segmental painful erythema. Mult Scler Relat Disord. 2017 Jan;11:43-44.
Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol. 2017 Dec;13(12):755-763.
Kümpfel T, Müller-Felber W, Rostasy K. Multiple Sklerose im Kindes- und Jugendalter : Komplex, chronisch, differenziert [Multiple sclerosis in childhood and adolescence : Complex, chronic and differentiated]. Nervenarzt. 2017 Dec;88(12):1377-1384.
Thaler FS, Laurent SA, Huber M, Mulazzani M, Dreyling M, Ködel U, Kümpfel T, Straube A, Meinl E, von Baumgarten L. Soluble TACI and soluble BCMA as biomarkers in primary central nervous system lymphoma. Neuro Oncol. 2017 Nov 29;19(12):1618-1627.
Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, Traboulsee A, Wolinsky JS, Arnold DL, Klingelschmitt G, Masterman D, Fontoura P, Belachew S, Chin P, Mairon N, Garren H, Kappos L; OPERA I and OPERA II Clinical Investigators. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med. 2017 Jan 19;376(3):221-234.
Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, Kümpfel T, Hohlfeld R, Krishnamoorthy G, Wekerle H. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):10719-10724.
Kyratsous NI, Bauer IJ, Zhang G, Pesic M, Bartholomäus I, Mues M, Fang P, Wörner M, Everts S, Ellwart JW, Watt JM, Potter BVL, Hohlfeld R, Wekerle H, Kawakami N. Visualizing context-dependent calcium signaling in encephalitogenic T cells in vivo by two-photon microscopy. Proc Natl Acad Sci U S A. 2017 Aug 1;114(31):E6381-E6389.
2016
Kawakami N. In vivo imaging in autoimmune diseases in the central nervous system. Allergol Int. 2016 Jul;65(3):235-42.
Gerdes LA, Held K, Beltrán E, Berking C, Prinz JC, Junker A, Tietze JK, Ertl-Wagner B, Straube A, Kümpfel T, Dornmair K, Hohlfeld R. CTLA4 as Immunological Checkpoint in the Development of Multiple Sclerosis. Ann Neurol. 2016 Aug;80(2):294-30
Kleiter I, Gahlen A, Borisow N, Fischer K, Wernecke KD, Wegner B, Hellwig K, Pache F, Ruprecht K, Havla J, Krumbholz M, Kümpfel T, Aktas O, Hartung HP, Ringelstein M, Geis C, Kleinschnitz C, Berthele A, Hemmer B, Angstwurm K, Stellmann JP, Schuster S, Stangel M, Lauda F, Tumani H, Mayer C, Zeltner L, Ziemann U, Linker R, Schwab M, Marziniak M, Then Bergh F, Hofstadt-van Oy U, Neuhaus O, Winkelmann A, Marouf W, Faiss J, Wildemann B, Paul F, Jarius S, Trebst C; Neuromyelitis Optica Study Group. Neuromyelitis optica: Evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016 Feb;79(2):206-16.
Jürgens T, Jafari M, Kreutzfeldt M, Bahn E, Brück W, Kerschensteiner M, Merkler D. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain. 2016 Jan;139(Pt 1):39-46.
Hohlfeld R, Schulze-Koops H. Cytotoxic T cells go awry in inclusion body myositis. Brain. 2016 May;139(Pt 5):1312-4.
Diem R, Molnar F, Beisse F, Gross N, Drüschler K, Heinrich SP, Joachimsen L, Rauer S, Pielen A, Sühs KW, Linker RA, Huchzermeyer C, Albrecht P, Hassenstein A, Aktas O, Guthoff T, Tonagel F, Kernstock C, Hartmann K, Kümpfel T, Hein K, van Oterendorp C, Grotejohann B, Ihorst G, Maurer J, Müller M, Volkmann M, Wildemann B, Platten M, Wick W, Heesen C, Schiefer U, Wolf S, Lagrèze WA. Treatment of optic neuritis with erythropoietin (TONE): a randomised, double-blind, placebo-controlled trial-study protocol. BMJ Open. 2016 Mar 1;6(3):e010956.
Vural A, Göçmen R, Kurne AT, Oğuz KK, Temuçin ÇM, Tan E, Karabudak R, Meinl E, Erdem Özdamar S. Fulminant Central Plus Peripheral Nervous System Demyelination without Antibodies to Neurofascin. Can J Neurol Sci. 2016 Jan;43(1):149-56.
Weil MT, Möbius W, Winkler A, Ruhwedel T, Wrzos C, Romanelli E, Bennett JL, Enz L, Goebels N, Nave KA, Kerschensteiner M, Schaeren-Wiemers N, Stadelmann C, Simons M. Loss of Myelin Basic Protein Function Triggers Myelin Breakdown in Models of Demyelinating Diseases. Cell Rep. 2016 Jul 12;16(2):314-322.
Höhne C, Schuh E, Kümpfel T, Straube A. Cryopyrin-associated periodic fever syndrome manifesting as Tolosa-Hunt syndrome. Cephalalgia. 2016 Dec;36(14):1392-1396.
Havla J, Warnke C, Derfuss T, Kappos L, Hartung HP, Hohlfeld R. Interdisciplinary Risk Management in the Treatment of Multiple Sclerosis. Dtsch Arztebl Int. 2016 Dec 26;113(51-52):879-886.
Sadovnick AD, Traboulsee AL, Bernales CQ, Ross JP, Forwell AL, Yee IM, Guillot-Noel L, Fontaine B, Cournu-Rebeix I, Alcina A, Fedetz M, Izquierdo G, Matesanz F, Hilven K, Dubois B, Goris A, Astobiza I, Alloza I, Antigüedad A, Vandenbroeck K, Akkad DA, Aktas O, Blaschke P, Buttmann M, Chan A, Epplen JT, Gerdes LA, Kroner A, Kubisch C, Kümpfel T, Lohse P, Rieckmann P, Zettl UK, Zipp F, Bertram L, Lill CM, Fernandez O, Urbaneja P, Leyva L, Alvarez-Cermeño JC, Arroyo R, Garagorri AM, García-Martínez A, Villar LM, Urcelay E, Malhotra S, Montalban X, Comabella M, Berger T, Fazekas F, Reindl M, Schmied MC, Zimprich A, Vilariño-Güell C. Analysis of Plasminogen Genetic Variants in Multiple Sclerosis Patients. G3 (Bethesda). 2016 Jul 7;6(7):2073-9.
Schankin CJ, Kästele F, Gerdes LA, Winkler T, Csanadi E, Högen T, Pellkofer H, Paulus W, Kümpfel T, Straube A. New-Onset Headache in Patients With Autoimmune Encephalitis Is Associated With anti-NMDA-Receptor Antibodies. Headache. 2016 Jun;56(6):995-1003.
Souren NY, Gerdes LA, Kümpfel T, Lutsik P, Klopstock T, Hohlfeld R, Walter J. Mitochondrial DNA Variation and Heteroplasmy in Monozygotic Twins Clinically Discordant for Multiple Sclerosis. Hum Mutat. 2016 Aug;37(8):765-75.
Hohlfeld R, Kümpfel T. Alemtuzumab and Multiple Sclerosis: Another Note of Caution. JAMA Neurol. 2016 Jun 1;73(6):637-8.
Sunderkötter C, Nast A, Worm M, Dengler R, Dörner T, Ganter H, Hohlfeld R, Melms A, Melzer N, Rösler K, Schmidt J, Sinnreich M, Walter MC, Wanschitz J, Wiendl H. Guidelines on dermatomyositis--excerpt from the interdisciplinary S2k guidelines on myositis syndromes by the German Society of Neurology. J Dtsch Dermatol Ges. 2016 Mar;14(3):321-38.
Schuh E, Berer K, Mulazzani M, Feil K, Meinl I, Lahm H, Krane M, Lange R, Pfannes K, Subklewe M, Gürkov R, Bradl M, Hohlfeld R, Kümpfel T, Meinl E, Krumbholz M. Features of Human CD3+CD20+ T Cells. J Immunol. 2016 Aug 15;197(4):1111-7.
Rommer PS, Dörner T, Freivogel K, Haas J, Kieseier BC, Kümpfel T, Paul F, Proft F, Schulze-Koops H, Schmidt E, Wiendl H, Ziemann U, Zettl UK; GRAID investigators. Safety and Clinical Outcomes of Rituximab Treatment in Patients with Multiple Sclerosis and Neuromyelitis Optica: Experience from a National Online Registry (GRAID). J Neuroimmune Pharmacol. 2016 Mar;11(1):1-8.
Wasser B, Pramanik G, Hess M, Klein M, Luessi F, Dornmair K, Bopp T, Zipp F, Witsch E. Increase of Alternatively Activated Antigen Presenting Cells in Active Experimental Autoimmune Encephalomyelitis. J Neuroimmune Pharmacol. 2016 Dec;11(4):721-732.
Zilkha-Falb R, Kaushansky N, Kawakami N, Ben-Nun A. Post-CNS-inflammation expression of CXCL12 promotes the endogenous myelin/neuronal repair capacity following spontaneous recovery from multiple sclerosis-like disease. J Neuroinflammation. 2016 Jan 8;13:7.
Melzer N, Ruck T, Fuhr P, Gold R, Hohlfeld R, Marx A, Melms A, Tackenberg B, Schalke B, Schneider-Gold C, Zimprich F, Meuth SG, Wiendl H. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J Neurol. 2016 Aug;263(8):1473-94.
Ayzenberg I, Schöllhammer J, Hoepner R, Hellwig K, Ringelstein M, Aktas O, Kümpfel T, Krumbholz M, Trebst C, Paul F, Pache F, Obermann M, Zeltner L, Schwab M, Berthele A, Jarius S, Kleiter I; Neuromyelitis Optica Study Group. Efficacy of glatiramer acetate in neuromyelitis optica spectrum disorder: a multicenter retrospective study. J Neurol. 2016 Mar;263(3):575-82.
Hohlfeld R, Dornmair K, Meinl E, Wekerle H. The search for the target antigens of multiple sclerosis, part 1: autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol. 2016 Feb;15(2):198-209.
Hohlfeld R, Kerschensteiner M. Antiglutamatergic therapy for multiple sclerosis? Lancet Neurol. 2016 Sep;15(10):1003-4.
Andlauer TF, Buck D, Antony G, Bayas A, Bechmann L, Berthele A, Chan A, Gasperi C, Gold R, Graetz C, Haas J, Hecker M, Infante-Duarte C, Knop M, Kümpfel T, Limmroth V, Linker RA, Loleit V, Luessi F, Meuth SG, Mühlau M, Nischwitz S, Paul F, Pütz M, Ruck T, Salmen A, Stangel M, Stellmann JP, Stürner KH, Tackenberg B, Then Bergh F, Tumani H, Warnke C, Weber F, Wiendl H, Wildemann B, Zettl UK, Ziemann U, Zipp F, Arloth J, Weber P, Radivojkov-Blagojevic M, Scheinhardt MO, Dankowski T, Bettecken T, Lichtner P, Czamara D, Carrillo-Roa T, Binder EB, Berger K, Bertram L, Franke A, Gieger C, Herms S, Homuth G, Ising M, Jöckel KH, Kacprowski T, Kloiber S, Laudes M, Lieb W, Lill CM, Lucae S, Meitinger T, Moebus S, Müller-Nurasyid M, Nöthen MM, Petersmann A, Rawal R, Schminke U, Strauch K, Völzke H, Waldenberger M, Wellmann J, Porcu E, Mulas A, Pitzalis M, Sidore C, Zara I, Cucca F, Zoledziewska M, Ziegler A, Hemmer B, Müller-Myhsok B. Novel multiple sclerosis susceptibility loci implicated in epigenetic regulation. Sci Adv. 2016 Jun 17;2(6):e1501678.
Spadaro M, Meinl E. Detection of Autoantibodies Against Myelin Oligodendrocyte Glycoprotein in Multiple Sclerosis and Related Diseases. Methods Mol Biol. 2016;1304:99-104.
Schwab N, Schneider-Hohendorf T, Pignolet B, Spadaro M, Görlich D, Meinl I, Windhagen S, Tackenberg B, Breuer J, Cantó E, Kümpfel T, Hohlfeld R, Siffrin V, Luessi F, Posevitz-Fejfár A, Montalban X, Meuth SG, Zipp F, Gold R, Du Pasquier RA, Kleinschnitz C, Jacobi A, Comabella M, Bertolotto A, Brassat D, Wiendl H. PML risk stratification using anti-JCV antibody index and L-selectin. Mult Scler. 2016 Jul;22(8):1048-60.
Kuhle J, Hardmeier M, Disanto G, Gugleta K, Ecsedi M, Lienert C, Amato MP, Baum K, Buttmann M, Bayas A, Brassat D, Brochet B, Confavreux C, Edan G, Färkkilä M, Fredrikson S, Frontoni M, D'Hooghe M, Hutchinson M, De Keyser J, Kieseier BC, Kümpfel T, Rio J, Polman C, Roullet E, Stolz C, Vass K, Wandinger KP, Kappos L; European Long-term Follow-up Study Group in Interferon β-1b in Secondary-progressive Multiple Sclerosis. A 10-year follow-up of the European multicenter trial of interferon β-1b in secondary-progressive multiple sclerosis. Mult Scler. 2016 Apr;22(4):533-43.
Schneider-Hohendorf T, Mohan H, Bien CG, Breuer J, Becker A, Görlich D, Kuhlmann T, Widman G, Herich S, Elpers C, Melzer N, Dornmair K, Kurlemann G, Wiendl H, Schwab N. CD8(+) T-cell pathogenicity in Rasmussen encephalitis elucidated by large-scale T-cell receptor sequencing. Nat Commun. 2016 Apr 4;7:11153.
Romanelli E, Merkler D, Mezydlo A, Weil MT, Weber MS, Nikić I, Potz S, Meinl E, Matznick FE, Kreutzfeldt M, Ghanem A, Conzelmann KK, Metz I, Brück W, Routh M, Simons M, Bishop D, Misgeld T, Kerschensteiner M. Myelinosome formation represents an early stage of oligodendrocyte damage in multiple sclerosis and its animal model. Nat Commun. 2016 Nov 16;7:13275.
Goldmann T, Wieghofer P, Jordão MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FM, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016 Jul;17(7):797-805.
Klotz L, Berthele A, Brück W, Chan A, Flachenecker P, Gold R, Haghikia A, Hellwig K, Hemmer B, Hohlfeld R, Korn T, Kümpfel T, Lang M, Limmroth V, Linker RA, Meier U, Meuth SG, Paul F, Salmen A, Stangel M, Tackenberg B, Tumani H, Warnke C, Weber MS, Ziemssen T, Zipp F, Wiendl H. Monitoring von Blutparametern unter verlaufsmodifizierender MS-Therapie : Substanzspezifische Relevanz und aktuelle Handlungsempfehlungen [Monitoring of blood parameters under course-modified MS therapy : Substance-specific relevance and current recommendations for action]. Nervenarzt. 2016 Jun;87(6):645-59.
Spadaro M, Gerdes LA, Krumbholz M, Ertl-Wagner B, Thaler FS, Schuh E, Metz I, Blaschek A, Dick A, Brück W, Hohlfeld R, Meinl E, Kümpfel T. Autoantibodies to MOG in a distinct subgroup of adult multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016 Jun 30;3(5):e257.
Kümpfel T, Gerdes LA, Heck C, Prüss H. Delayed diagnosis of extraovarian teratoma in relapsing anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. 2016 Jun 16;3(4):e250.
Rühl G, Niedl AG, Patronov A, Siewert K, Pinkert S, Kalemanov M, Friese MA, Attfield KE, Antes I, Hohlfeld R, Dornmair K. Multiple sclerosis: Molecular mimicry of an antimyelin HLA class I restricted T-cell receptor. Neurol Neuroimmunol Neuroinflamm. 2016 May 17;3(4):e241.
Kappos L, Edan G, Freedman MS, Montalbán X, Hartung HP, Hemmer B, Fox EJ, Barkhof F, Schippling S, Schulze A, Pleimes D, Pohl C, Sandbrink R, Suarez G, Wicklein EM; BENEFIT Study Group. The 11-year long-term follow-up study from the randomized BENEFIT CIS trial. Neurology. 2016 Sep 6;87(10):978-87.
Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, Saidha S, Martinez-Lapiscina EH, Lagreze WA, Schuman JS, Villoslada P, Calabresi P, Balcer L, Petzold A, Green AJ, Paul F, Brandt AU, Albrecht P; IMSVISUAL consortium. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016 Jun 14;86(24):2303-9.
Lopez-Chiriboga AS, Komorowski L, Kümpfel T, Probst C, Hinson SR, Pittock SJ, McKeon A. Metabotropic glutamate receptor type 1 autoimmunity: Clinical features and treatment outcomes. Neurology. 2016 Mar 15;86(11):1009-13.
Brändle SM, Obermeier B, Senel M, Bruder J, Mentele R, Khademi M, Olsson T, Tumani H, Kristoferitsch W, Lottspeich F, Wekerle H, Hohlfeld R, Dornmair K. Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc Natl Acad Sci U S A. 2016 Jul 12;113(28):7864-9.
Gross CC, Schulte-Mecklenbeck A, Rünzi A, Kuhlmann T, Posevitz-Fejfár A, Schwab N, Schneider-Hohendorf T, Herich S, Held K, Konjević M, Hartwig M, Dornmair K, Hohlfeld R, Ziemssen T, Klotz L, Meuth SG, Wiendl H. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc Natl Acad Sci U S A. 2016 May 24;113(21):E2973-82.
Fujikawa Y, Roma LP, Sobotta MC, Rose AJ, Diaz MB, Locatelli G, Breckwoldt MO, Misgeld T, Kerschensteiner M, Herzig S, Müller-Decker K, Dick TP. Mouse redox histology using genetically encoded probes. Sci Signal. 2016 Mar 15;9(419):rs1.
2015
Ligocki AJ, Rivas JR, Rounds WH, Guzman AA, Li M, Spadaro M, Lahey L, Chen D, Henson PM, Graves D, Greenberg BM, Frohman EM, Ward ES, Robinson W, Meinl E, White CL 3rd, Stowe AM, Monson NL. A Distinct Class of Antibodies May Be an Indicator of Gray Matter Autoimmunity in Early and Established Relapsing Remitting Multiple Sclerosis Patients. ASN Neuro. 2015 Oct 21;7(5):1759091415609613.
Spadaro M, Gerdes LA, Mayer MC, Ertl-Wagner B, Laurent S, Krumbholz M, Breithaupt C, Högen T, Straube A, Giese A, Hohlfeld R, Lassmann H, Meinl E, Kümpfel T. Histopathology and clinical course of MOG-antibody-associated encephalomyelitis. Ann Clin Transl Neurol. 2015 Mar;2(3):295-301.
Breckwoldt MO, Wittmann C, Misgeld T, Kerschensteiner M, Grabher C. Redox imaging using genetically encoded redox indicators in zebrafish and mice. Biol Chem. 2015 May;396(5):511-22.
Reinholz M, Heppt MV, Hoffmann FS, Lummel N, Ruzicka T, Lehmann P, Berking C. Transient memory impairment and transient global amnesia induced by photodynamic therapy. Br J Dermatol. 2015 Nov;173(5):1258-62.
Lindner M, Thümmler K, Arthur A, Brunner S, Elliott C, McElroy D, Mohan H, Williams A, Edgar JM, Schuh C, Stadelmann C, Barnett SC, Lassmann H, Mücklisch S, Mudaliar M, Schaeren-Wiemers N, Meinl E, Linington C. Fibroblast growth factor signalling in multiple sclerosis: inhibition of myelination and induction of pro-inflammatory environment by FGF9. Brain. 2015 Jul;138(Pt 7):1875-93.
Schreiner B, Romanelli E, Liberski P, Ingold-Heppner B, Sobottka-Brillout B, Hartwig T, Chandrasekar V, Johannssen H, Zeilhofer HU, Aguzzi A, Heppner F, Kerschensteiner M, Becher B. Astrocyte Depletion Impairs Redox Homeostasis and Triggers Neuronal Loss in the Adult CNS. Cell Rep. 2015 Sep 1;12(9):1377-84.
Jacobi A, Loy K, Schmalz AM, Hellsten M, Umemori H, Kerschensteiner M, Bareyre FM. FGF22 signaling regulates synapse formation during post-injury remodeling of the spinal cord. EMBO J. 2015 May 5;34(9):1231-43.
Dankowski T, Buck D, Andlauer TF, Antony G, Bayas A, Bechmann L, Berthele A, Bettecken T, Chan A, Franke A, Gold R, Graetz C, Haas J, Hecker M, Herms S, Infante-Duarte C, Jöckel KH, Kieseier BC, Knier B, Knop M, Kümpfel T, Lichtner P, Lieb W, Lill CM, Limmroth V, Linker RA, Loleit V, Meuth SG, Moebus S, Müller-Myhsok B, Nischwitz S, Nöthen MM, Paul F, Pütz M, Ruck T, Salmen A, Stangel M, Stellmann JP, Strauch K, Stürner KH, Tackenberg B, Then Bergh F, Tumani H, Waldenberger M, Weber F, Wiendl H, Wildemann B, Zettl UK, Ziemann U, Zipp F, Hemmer B, Ziegler A; German Competence Network for Multiple Sclerosis (KKNMS). Successful Replication of GWAS Hits for Multiple Sclerosis in 10,000 Germans Using the Exome Array. Genet Epidemiol. 2015 Dec;39(8):601-8.
Held K, Beltrán E, Moser M, Hohlfeld R, Dornmair K. T-cell receptor repertoire of human peripheral CD161hiTRAV1-2+ MAIT cells revealed by next generation sequencing and single cell analysis. Hum Immunol. 2015 Sep;76(9):607-14.
Havla J, Kümpfel T, Hohlfeld R. Immuntherapie der multiplen Sklerose : Überblick und Update [Immunotherapies for multiple sclerosis : review and update]. Internist (Berl). 2015 Apr;56(4):432-45.
Arakawa A, Siewert K, Stöhr J, Besgen P, Kim SM, Rühl G, Nickel J, Vollmer S, Thomas P, Krebs S, Pinkert S, Spannagl M, Held K, Kammerbauer C, Besch R, Dornmair K, Prinz JC. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015 Dec 14;212(13):2203-12.
Hoffmann FS, Kuhn PH, Laurent SA, Hauck SM, Berer K, Wendlinger SA, Krumbholz M, Khademi M, Olsson T, Dreyling M, Pfister HW, Alexander T, Hiepe F, Kümpfel T, Crawford HC, Wekerle H, Hohlfeld R, Lichtenthaler SF, Meinl E. The immunoregulator soluble TACI is released by ADAM10 and reflects B cell activation in autoimmunity. J Immunol. 2015 Jan 15;194(2):542-52.
Lill CM, Luessi F, Alcina A, Sokolova EA, Ugidos N, de la Hera B, Guillot-Noël L, Malhotra S, Reinthaler E, Schjeide BM, Mescheriakova JY, Mashychev A, Wohlers I, Akkad DA, Aktas O, Alloza I, Antigüedad A, Arroyo R, Astobiza I, Blaschke P, Boyko AN, Buttmann M, Chan A, Dörner T, Epplen JT, Favorova OO, Fedetz M, Fernández O, García-Martínez A, Gerdes LA, Graetz C, Hartung HP, Hoffjan S, Izquierdo G, Korobko DS, Kroner A, Kubisch C, Kümpfel T, Leyva L, Lohse P, Malkova NA, Montalban X, Popova EV, Rieckmann P, Rozhdestvenskii AS, Schmied C, Smagina IV, Tsareva EY, Winkelmann A, Zettl UK, Binder H, Cournu-Rebeix I, Hintzen R, Zimprich A, Comabella M, Fontaine B, Urcelay E, Vandenbroeck K, Filipenko M, Matesanz F, Zipp F, Bertram L. Genome-wide significant association with seven novel multiple sclerosis risk loci. J Med Genet. 2015 Dec;52(12):848-55.
Pröbstel AK, Rudolf G, Dornmair K, Collongues N, Chanson JB, Sanderson NS, Lindberg RL, Kappos L, de Seze J, Derfuss T. Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype. J Neuroinflammation. 2015 Mar 8;12:46.
Schrewe L, Lill CM, Liu T, Salmen A, Gerdes LA, Guillot-Noel L, Akkad DA, Blaschke P, Graetz C, Hoffjan S, Kroner A, Demir S, Böhme A, Rieckmann P, ElAli A, Hagemann N, Hermann DM, Cournu-Rebeix I, Zipp F, Kümpfel T, Buttmann M, Zettl UK, Fontaine B, Bertram L, Gold R, Chan A. Investigation of sex-specific effects of apolipoprotein E on severity of EAE and MS. J Neuroinflammation. 2015 Dec 16;12:234.
Wuhrer M, Selman MH, McDonnell LA, Kümpfel T, Derfuss T, Khademi M, Olsson T, Hohlfeld R, Meinl E, Krumbholz M. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J Neuroinflammation. 2015 Dec 18;12:235.
Flytzani S, Guerreiro-Cacais AO, N'diaye M, Lindner M, Linington C, Meinl E, Stridh P, Jagodic M, Olsson T. MOG-induced experimental autoimmune encephalomyelitis in the rat species triggers anti-neurofascin antibody response that is genetically regulated. J Neuroinflammation. 2015 Oct 29;12:194.
Hoffmann FS, Hofereiter J, Rübsamen H, Melms J, Schwarz S, Faber H, Weber P, Pütz B, Loleit V, Weber F, Hohlfeld R, Meinl E, Krumbholz M. Fingolimod induces neuroprotective factors in human astrocytes. J Neuroinflammation. 2015 Sep 30;12:184.
Krumbholz M, Hofstadt-van Oy U, Angstwurm K, Kleiter I, Jarius S, Paul F, Aktas O, Buchholz G, Kern P, Straube A, Kümpfel T. Very late-onset neuromyelitis optica spectrum disorder beyond the age of 75. J Neurol. 2015 May;262(5):1379-84.
Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015 Apr;14(4):406-19.
Warnke C, Stettner M, Lehmensiek V, Dehmel T, Mausberg AK, von Geldern G, Gold R, Kümpfel T, Hohlfeld R, Mäurer M, Stangel M, Straeten V, Limmroth V, Weber T, Kleinschnitz C, Wattjes MP, Svenningsson A, Olsson T, Hartung HP, Hermsen D, Tumani H, Adams O, Kieseier BC. Natalizumab exerts a suppressive effect on surrogates of B cell function in blood and CSF. Mult Scler. 2015 Jul;21(8):1036-44.
Laurent SA, Hoffmann FS, Kuhn PH, Cheng Q, Chu Y, Schmidt-Supprian M, Hauck SM, Schuh E, Krumbholz M, Rübsamen H, Wanngren J, Khademi M, Olsson T, Alexander T, Hiepe F, Pfister HW, Weber F, Jenne D, Wekerle H, Hohlfeld R, Lichtenthaler SF, Meinl E. γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. 2015 Jun 11;6:7333.
Kolber P, Luessi F, Meuth SG, Klotz L, Korn T, Trebst C, Tackenberg B, Kieseier B, Kümpfel T, Fleischer V, Tumani H, Wildemann B, Lang M, Flachenecker P, Meier U, Brück W, Limmroth V, Haghikia A, Hartung HP, Stangel M, Hohlfeld R, Hemmer B, Gold R, Wiendl H, Zipp F. Aktuelles zur Therapieumstellung bei Multipler Sklerose [Current aspects of therapy conversion for multiple sclerosis]. Nervenarzt. 2015 Oct;86(10):1236-47.
Hoffmann F, Kraft A, Heigl F, Mauch E, Koehler J, Harms L, Kümpfel T, Köhler W, Klingel R, Fassbender C, Schimrigk S. Tryptophan-Immunadsorption bei Multipler Sklerose und Neuromyelitis optica : Therapieoption bei akuten Schüben in der Schwangerschaft und Stillphase [Tryptophan immunoadsorption for multiple sclerosis and neuromyelitis optica: therapy option for acute relapses during pregnancy and breastfeeding]. Nervenarzt. 2015 Feb;86(2):179-86.
Hohlfeld R, Wekerle H. Multiple Sklerose und Mikrobiota. Vom Genom zum Metagenom? [Multiple sclerosis and microbiota. From genome to metagenome?]. Nervenarzt. 2015 Aug;86(8):925-33.
Jacobi A, Bareyre FM. Regulation of axonal remodeling following spinal cord injury. Neural Regen Res. 2015 Oct;10(10):1555-7.
Held K, Bhonsle-Deeng L, Siewert K, Sato W, Beltrán E, Schmidt S, Rühl G, Ng JK, Engerer P, Moser M, Klinkert WE, Babbe H, Misgeld T, Wekerle H, Laplaud DA, Hohlfeld R, Dornmair K. αβ T-cell receptors from multiple sclerosis brain lesions show MAIT cell-related features. Neurol Neuroimmunol Neuroinflamm. 2015 May 7;2(4):e107.
Schuh E, Lohse P, Ertl-Wagner B, Witt M, Krumbholz M, Frankenberger M, Gerdes LA, Hohlfeld R, Kümpfel T. Expanding spectrum of neurologic manifestations in patients with NLRP3 low-penetrance mutations. Neurol Neuroimmunol Neuroinflamm. 2015 May 14;2(4):e109.
Kappos L, O'Connor P, Radue EW, Polman C, Hohlfeld R, Selmaj K, Ritter S, Schlosshauer R, von Rosenstiel P, Zhang-Auberson L, Francis G. Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology. 2015 Apr 14;84(15):1582-91.
Miller DH, Fox RJ, Phillips JT, Hutchinson M, Havrdova E, Kita M, Wheeler-Kingshott CA, Tozer DJ, MacManus DG, Yousry TA, Goodsell M, Yang M, Zhang R, Viglietta V, Dawson KT; CONFIRM study investigators. Effects of delayed-release dimethyl fumarate on MRI measures in the phase 3 CONFIRM study. Neurology. 2015 Mar 17;84(11):1145-52.
Doppler K, Appeltshauser L, Krämer HH, Ng JK, Meinl E, Villmann C, Brophy P, Dib-Hajj SD, Waxman SG, Weishaupt A, Sommer C. Contactin-1 and Neurofascin-155/-186 Are Not Targets of Auto-Antibodies in Multifocal Motor Neuropathy. PLoS One. 2015 Jul 28;10(7):e0134274.